Abstract
Introduction
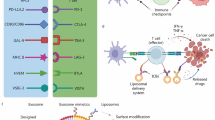
Immune checkpoint blockade (ICB) is a promising strategy for cancer treatment and has generated remarkable clinical results against multiple malignancies. Exploration of new technical approaches to further boost the therapeutic efficacy of ICB is of potential medical importance. In this study, we designed a novel nanotherapeutics for ICB immunotherapy.
Methods
CTLA-4 aptamers were conjugated to the surface of albumin nanoparticle to construct an aptamer-modified nanostructure (Apt-NP). To improve ICB efficacy, fexofenadine (FEXO), an antihistamine, was encapsulated into Apt-NP to make a drug-loaded nanoparticle (Apt-NP-FEXO). The antitumor efficacies of Apt-NP and Apt-NP-FEXO were evaluated in vitro and in vivo.
Results
Apt-NP and Apt-NP-FEXO had average diameters of 149 nm and 159 nm, respectively. Similar to free CTLA-4 aptamers, Apt-modified NPs could selectively bind with CTLA-4 positive cells and improve lymphocyte-mediated antitumor cytotoxicity in vitro. In animal studies, compared with free CTLA-4 aptamer, Apt-NP significantly enhanced antitumor immunity. Moreover, Apt-NP-FEXO further improved antitumor efficacy vs. Apt-NP in vivo.
Conclusion
The results suggest that Apt-NP-FEXO represents a novel strategy to improve ICB outcome and may have application potential in cancer immunotherapy.










Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study is included in this published article.
References
Abiri N et al (2018) Assessment of the immunogenicity of residual host cell protein impurities of OsrHSA. PLoS One 13(3):e0193339
Albanese A, Tang PS, Chan WC (2012) The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng 14:1–16
An Y et al (2022) Novel complex of PD-L1 aptamer and albumin enhances antitumor efficacy in vivo. Molecules 27(5):1482
Binnewies M et al (2018) Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 24(5):541–550
Buff MC et al (2010) Dependence of aptamer activity on opposed terminal extensions: improvement of light-regulation efficiency. Nucleic Acids Res 38(6):2111–2118
Chen R et al (2017) Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol 35(19):2125–2132
Cohen MG et al (2010) First clinical application of an actively reversible direct factor IXa inhibitor as an anticoagulation strategy in patients undergoing percutaneous coronary intervention. Circulation 122(6):614–622
Fritz I, Wagner P, Olsson H (2021) Improved survival in several cancers with use of H1-antihistamines desloratadine and loratadine. Transl Oncol 14(4):101029
Gao T, Pei R (2020) Isolation of DNA aptamer targeting PD-1 with an antitumor immunotherapy effect. ACS Appl Bio Mater 3(10):7080–7086
Grubczak K et al (2021) Differential Response of MDA-MB-231 and MCF-7 Breast Cancer Cells to In Vitro Inhibition with CTLA-4 and PD-1 through Cancer-Immune Cells Modified Interactions. Cells 10(8):2044
Guo P (2010) The emerging field of RNA nanotechnology. Nat Nanotechnol 5(12):833–842
He F et al (2020) Aptamer-based targeted drug delivery systems: current potential and challenges. Curr Med Chem 27(13):2189–2219
Hodi FS et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363(8):711–723
Huang BT et al (2017) A CTLA-4 antagonizing DNA aptamer with antitumor effect. Mol Ther Nucleic Acids 8:520–528
Jaffe GJ et al (2021) C5 inhibitor avacincaptad pegol for geographic atrophy due to age-related macular degeneration: a randomized pivotal phase 2/3 trial. Ophthalmology 128(4):576–586
Jutel M et al (2001) Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature 413(6854):420–425
Kobayashi H, Watanabe R, Choyke PL (2013) Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics 4(1):81–89
Kundranda MN, Niu J (2015) Albumin-bound paclitaxel in solid tumors: clinical development and future directions. Drug Des Devel Ther 9:3767–3777
Lambert JM, Chari RV (2014) Ado-trastuzumab Emtansine (T-DM1): an antibody-drug conjugate (ADC) for HER2-positive breast cancer. J Med Chem 57(16):6949–6964
Li H et al (2022) The allergy mediator histamine confers resistance to immunotherapy in cancer patients via activation of the macrophage histamine receptor H1. Cancer Cell 40(1):36–52 (e9)
Morad G et al (2021) Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 184(21):5309–5337
Morita Y et al (2018) Aptamer therapeutics in cancer: current and future. Cancers (basel) 10(3):80
Natarajan JV et al (2014) Sustained-release from nanocarriers: a review. J Control Release 193:122–138
Ogloblina AM et al (2020) Toward G-quadruplex-based anticancer agents: biophysical and biological studies of novel AS1411 derivatives. Int J Mol Sci 21(20):7781
Ozer I et al (2022) PEG-like brush polymer conjugate of RNA aptamer that shows reversible anticoagulant activity and minimal immune response. Adv Mater 34(10):e2107852
Prodeus A et al (2015) Targeting the PD-1/PD-L1 immune evasion axis with DNA aptamers as a novel therapeutic strategy for the treatment of disseminated cancers. Mol Ther Nucleic Acids 4:e237
Sarasola MP et al (2021) Histamine in cancer immunology and immunotherapy. Current status and new perspectives. Pharmacol Res Perspect 9(5):e00778
Shao XR et al (2015) Independent effect of polymeric nanoparticle zeta potential/surface charge, on their cytotoxicity and affinity to cells. Cell Prolif 48(4):465–474
Shi H, Lan J, Yang J (2020) Mechanisms of resistance to checkpoint blockade therapy. Adv Exp Med Biol 1248:83–117
Stein CA, Castanotto D (2017) FDA-approved oligonucleotide therapies in 2017. Mol Ther 25(5):1069–1075
Sterle HA et al (2019) Immunomodulatory role of histamine H4 receptor in breast cancer. Br J Cancer 120(1):128–138
Sung H et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Wan LY et al (2019) An exploration of aptamer internalization mechanisms and their applications in drug delivery. Expert Opin Drug Deliv 16(3):207–218
Wang Z, Duan Y, Duan Y (2018) Application of polydopamine in tumor targeted drug delivery system and its drug release behavior. J Control Release 290:56–74
Wei SC, Duffy CR, Allison JP (2018) Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 8(9):1069–1086
Yoshitomi T et al (2020) Binding and structural properties of DNA aptamers with VEGF-A-mimic activity. Mol Ther Nucleic Acids 19:1145–1152
Yu Z et al (2020) Targeted treatment of colon cancer with aptamer-guided albumin nanoparticles loaded with docetaxel. Int J Nanomedicine 15:6737–6748
Zhu G, Chen X (2018) Aptamer-based targeted therapy. Adv Drug Deliv Rev 134:65–78
Funding
This work was supported by the Ministry of Science and Technology (2017YFA0205504), and the Tianjin Science and Technology Plan Project (22JCQNJC01590).
Author information
Authors and Affiliations
Contributions
XDY and FJY designed the research. FJY, YCA, XLL and ZY carried out the experiments and collected data. XDL provided technical expertise and support. FJY wrote the original manuscript. XDY revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and consent to participate
PBMC was isolated from healthy donors. All donors were informed of the purpose of the experiment and required to sign an informed consent form. The procedure was approved by the Ethics Committee at Chinese Academy of Medical Sciences and Peking Union Medical College. The animal study was approved by the Ethical Committee of the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences. All animal procedures were approved by the committee on the Animal Care and Use of the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences and Peking Union Medical College and performed according to the Institutional Animal Care and Use guidelines.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yao, F., An, Y., Lai, X. et al. Novel nanotherapeutics for cancer immunotherapy by CTLA-4 aptamer-functionalized albumin nanoparticle loaded with antihistamine. J Cancer Res Clin Oncol 149, 7515–7527 (2023). https://doi.org/10.1007/s00432-023-04698-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-04698-y