Abstract
Purpose
In breast cancer management not only mortality and surgical morbidity measurements are important but also patient satisfaction indexes. The authors evaluated the satisfaction and health-related quality of life (HRQOL) using the breast-conserving therapy (BCT) and breast reduction (BR) modules of BREAST-Q®.
Methods
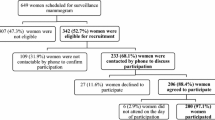
This is a cross-sectional study that analyzed breast cancer patients consecutively submitted to breast surgery between January 2011 and April 2018 using two modules of BREAST-Q®. 968 patients were contacted and 232 answers were gathered: 171 patients submitted to oncoplastic level 1 surgery answered the BCT module and 61 submitted to oncoplastic level 2 surgery answered the BR module. Clinical data were retrieved from patients’ medical records.
Results
Among the 232 questionnaires received, the median scores for psychosocial well-being, sexual well-being and (postoperative) satisfaction with breasts for BCT and BR modules were, respectively, 77.0 and 73.5 (p = 0.17); 62.0 and 53.0 (p = 0.14); 72.0 and 66.0 (p = 0.66). The median of adverse effects of radiation in the BCT module was 87.0. The median satisfaction with outcome in the BR module was 86.0. Both groups of patients revealed high scores of satisfaction with care. For the BCT patients, satisfaction with breasts strongly correlated with sexual well-being and was moderately correlated with psychosocial and physical well-being. For the BR patients, the satisfaction with outcome strongly correlated with satisfaction with medical team and moderately correlated with the remaining scales.
Conclusion
Both oncoplastic surgery levels yielded similar satisfaction outcomes when assessed using BCT and BR modules of BREAST-Q®.



Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to patient privacy protection.
Abbreviations
- BC:
-
Breast cancer
- BCT:
-
Breast-conserving therapy
- BMI:
-
Body mass index
- BR:
-
Breast reduction
- BS:
-
Breast surgery
- CHUSJ:
-
Centro Hospitalar Universitário de São João
- CI:
-
Confidence interval
- HRQOL:
-
Health-related quality of life
- MA:
-
Mastectomy alone
- MWBR:
-
Mastectomy with breast reconstruction
- NA:
-
Not applicable
- NAC:
-
Nipple-areola complex
- PRO:
-
Patient-reported outcomes
- PROMs:
-
Patient-reported outcomes measures
- PhyWB:
-
Physical well-being
- PsyWB:
-
Psychosocial well-being
- QC:
-
Quality of care
- SWB:
-
Satisfaction with breasts
- SexWB:
-
Sexual well-being
- SWI:
-
Satisfaction with information
- SWMT:
-
Satisfaction with the medical team
- SWO:
-
Satisfaction with the outcome
- SWOS:
-
Satisfaction with office staff
- SWS:
-
Satisfaction with the surgeon
- WLE:
-
Wide local excision
References
Acea-Nebril B, Cereijo-Garea C, Garcia-Novoa A, Bouzon-Alejandro A, Mosquera-Oses J (2020) Breast-Q 15 prospective study: oncoplastic breast reduction improve quality of live for women with macromastia. Breast J 26(9):1890–1892. https://doi.org/10.1111/tbj.13836
Acea-Nebril B, Cereijo-Garea C, Garcia-Novoa A, Varela-Lamas C, Builes-Ramirez S, Bouzon-Alejandro A, Mosquera-Oses J (2017) The role of oncoplastic breast reduction in the conservative management of breast cancer: Complications, survival, and quality of life. J Surg Oncol 115(6):679–686. https://doi.org/10.1002/jso.24550
Bazzarelli A, Baker L, Petrcich W, Zhang J, Arnaout A (2020) Patient satisfaction following level II Oncoplastic breast surgery: a comparison with mastectomy utililizing the breast-Q questionnaire will be published in surgical oncology. Surg Oncol 35:556–559. https://doi.org/10.1016/j.suronc.2020.11.001
Biganzoli L, Marotti L, Hart CD, Cataliotti L, Cutuli B, Kuhn T, Mansel RE, Ponti A, Poortmans P, Regitnig P, van der Hage JA, Wengstrom Y, Rosselli Del Turco M (2017) Quality indicators in breast cancer care: an update from the EUSOMA working group. Eur J Cancer 86:59–81. https://doi.org/10.1016/j.ejca.2017.08.017
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Cabral IV, da Silva Garcia E, Sobrinho RN, Pinto NLL, Juliano Y, Veiga-Filho J, Ferreira LM, Veiga DF (2018) Use of the BREAST-Q survey in the prospective evaluation of reduction mammaplasty outcomes. Aesthetic Plast Surg 42(2):388–395. https://doi.org/10.1007/s00266-017-1009-6
Cano SJ, Klassen AF, Scott AM, Cordeiro PG, Pusic AL (2012) The BREAST-Q: further validation in independent clinical samples. Plast Reconstr Surg 129(2):293–302. https://doi.org/10.1097/PRS.0b013e31823aec6b
Cano SJ, Klassen AF, Scott AM, Pusic AL (2013) A closer look at the BREAST-Q((c)). Clin Plast Surg 40(2):287–296. https://doi.org/10.1016/j.cps.2012.12.002
Chang E, Johnson N, Webber B, Booth J, Rahhal D, Gannett D, Johnson W, Franzini D, Zegzula H (2004) Bilateral reduction mammoplasty in combination with lumpectomy for treatment of breast cancer in patients with macromastia. Am J Surg 187(5):647–650. https://doi.org/10.1016/j.amjsurg.2004.01.002
Christiansen P, Carstensen SL, Ejlertsen B, Kroman N, Offersen B, Bodilsen A, Jensen MB (2018) Breast conserving surgery versus mastectomy: overall and relative survival-a population based study by the Danish breast cancer cooperative group (DBCG). Acta Oncol 57(1):19–25. https://doi.org/10.1080/0284186X.2017.1403042
Clough KB, Kaufman GJ, Nos C, Buccimazza I, Sarfati IM (2010) Improving breast cancer surgery: a classification and quadrant per quadrant atlas for oncoplastic surgery. Ann Surg Oncol 17(5):1375–1391. https://doi.org/10.1245/s10434-009-0792-y
Cogliandro A, Barone M, Cassotta G, Tenna S, Cagli B, Persichetti P (2017) Patient satisfaction and clinical outcomes following 414 breast reductions: application of BREAST-Q. Aesthetic Plast Surg 41(2):245–249. https://doi.org/10.1007/s00266-016-0774-y
Cohen WA, Homel P, Patel NP (2016a) Does time affect patient satisfaction and health-related quality of life after reduction mammoplasty? Eplasty 16:e7
Cohen WA, Mundy LR, Ballard TN, Klassen A, Cano SJ, Browne J, Pusic AL (2016b) The BREAST-Q in surgical research: a review of the literature 2009–2015. J Plast Reconstr Aesthet Surg 69(2):149–162. https://doi.org/10.1016/j.bjps.2015.11.013
Coriddi M, Nadeau M, Taghizadeh M, Taylor A (2013) Analysis of satisfaction and well-being following breast reduction using a validated survey instrument: the BREAST-Q. Plast Reconstr Surg 132(2):285–290. https://doi.org/10.1097/PRS.0b013e31829587b5
Correa MPD, Dornelas MT, de Carvalho EN, Barra A, Venturelli EP Jr, Correa LD, Chaoubah A (2018) Assessment of quality of life in patients who underwent breast reduction using BREAST-Q. J Plast Reconstr Aesthet Surg 71(6):929–931. https://doi.org/10.1016/j.bjps.2018.02.010
Dahlback C, Ullmark JH, Rehn M, Ringberg A, Manjer J (2017) Aesthetic result after breast-conserving therapy is associated with quality of life several years after treatment. Swedish women evaluated with BCCT.core and BREAST-Q. Breast Cancer Res Treat 164(3):679–687. https://doi.org/10.1007/s10549-017-4306-5
de Boniface J, Szulkin R, Johansson ALV (2021) Survival after breast conservation vs mastectomy adjusted for comorbidity and socioeconomic status: a Swedish national 6-year follow-up of 48986 women. JAMA Surg 156(7):628–637. https://doi.org/10.1001/jamasurg.2021.1438
Ettinger RE, Agarwal S, Izenberg PH, Beil RJ, Sherick DG (2016) Bilateral reduction mammaplasty as an oncoplastic technique for the management of early-stage breast cancer in women with macromastia. Eplasty 16:e5
Fuzesi S, Cano SJ, Klassen AF, Atisha D, Pusic AL (2017) Validation of the electronic version of the BREAST-Q in the army of women study. Breast 33:44–49. https://doi.org/10.1016/j.breast.2017.02.015
Gonzalez MA, Glickman LT, Aladegbami B, Simpson RL (2012) Quality of life after breast reduction surgery: a 10-year retrospective analysis using the Breast Q questionnaire: does breast size matter? Ann Plast Surg 69(4):361–363. https://doi.org/10.1097/SAP.0b013e31824a218a
Hopwood P, Haviland J, Mills J, Sumo G, Bliss JM, S. T. M. Group (2007) The impact of age and clinical factors on quality of life in early breast cancer: an analysis of 2208 women recruited to the UK START Trial (Standardisation of breast radiotherapy trial). Breast 16(3):241–251. https://doi.org/10.1016/j.breast.2006.11.003
Klassen AF, Dominici L, Fuzesi S, Cano SJ, Atisha D, Locklear T, Gregorowitsch ML, Tsangaris E, Morrow M, King T, Pusic AL (2020) Development and validation of the BREAST-Q breast-conserving therapy module. Ann Surg Oncol 27(7):2238–2247. https://doi.org/10.1245/s10434-019-08195-w
Klassen AF, Pusic AL, Scott A, Klok J, Cano SJ (2009) Satisfaction and quality of life in women who undergo breast surgery: a qualitative study. BMC Womens Health 9:11. https://doi.org/10.1186/1472-6874-9-11
Koppiker CB, Noor AU, Dixit S, Busheri L, Sharan G, Dhar U, Allampati HK, Nare S (2019) Extreme oncoplastic surgery for multifocal/multicentric and locally advanced breast cancer. Int J Breast Cancer 2019:4262589. https://doi.org/10.1155/2019/4262589
Lagendijk M, van Egdom LSE, Richel C, van Leeuwen N, Verhoef C, Lingsma HF, Koppert LB (2018) Patient reported outcome measures in breast cancer patients. Eur J Surg Oncol 44(7):963–968. https://doi.org/10.1016/j.ejso.2018.03.009
Liu LQ, Branford OA, Mehigan S (2018) BREAST-Q measurement of the patient perspective in oncoplastic breast surgery: a systematic review. Plast Reconstr Surg Glob Open 6(8):e1904. https://doi.org/10.1097/GOX.0000000000001904
Mundy LR, Homa K, Klassen AF, Pusic AL, Kerrigan CL (2017) Understanding the health burden of macromastia: normative data for the BREAST-Q reduction module. Plast Reconstr Surg 139(4):846e–853e. https://doi.org/10.1097/PRS.0000000000003171
O’Connell RL, DiMicco R, Khabra K, O’Flynn EA, deSouza N, Roche N, Barry PA, Kirby AM, Rusby JE (2016) Initial experience of the BREAST-Q breast-conserving therapy module. Breast Cancer Res Treat 160(1):79–89. https://doi.org/10.1007/s10549-016-3966-x
Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ (2009) Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg 124(2):345–353. https://doi.org/10.1097/PRS.0b013e3181aee807
Schnur JB, Ouellette SC, Dilorenzo TA, Green S, Montgomery GH (2011) A qualitative analysis of acute skin toxicity among breast cancer radiotherapy patients. Psychooncology 20(3):260–268. https://doi.org/10.1002/pon.1734
Stein MJ, Karir A, Arnaout A, Roberts A, Cordeiro E, Zhang T, Zhang J (2020) Quality-of-life and surgical outcomes for breast cancer patients treated with therapeutic reduction mammoplasty versus mastectomy with immediate reconstruction. Ann Surg Oncol 27(11):4502–4512. https://doi.org/10.1245/s10434-020-08574-8
Stolpner I, Heil J, Feisst M, Karsten MM, Weber WP, Blohmer JU, Forster T, Golatta M, Schutz F, Sohn C, Hennigs A (2019a) Clinical validation of the BREAST-Q breast-conserving therapy module. Ann Surg Oncol 26(9):2759–2767. https://doi.org/10.1245/s10434-019-07456-y
Stolpner I, Heil J, Hennigs A (2019b) ASO Author Reflections: The BREAST-Q BCT Module and Its Use in Clinical Practice. Ann Surg Oncol 26(Suppl 3):788–789. https://doi.org/10.1245/s10434-019-07939-y
Acknowledgment
The authors would like to thank the Memorial Sloan Kettering Cancer Center for providing the modules of Breast-Conserving Therapy and Reduction/Mastopexy of BREAST-Q®. We also would like to thank Beatriz Alves for assisting and reviewing the English scientific writing
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
PCA: Data collection, analysis and interpretation, statistical analysis, manuscript preparation and editing. PB: Statistical analysis and manuscript review. PCS: Manuscript review. OF, CS, MA, MH, AJ, GD: Surgical procedures and manuscript review. FJL: Study design, surgical procedures, data collection, manuscript review and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of CHUSJ (CE 322–19).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pinto, C.A., Peleteiro, B., Pinto, C.S. et al. Breast cancer patient-reported outcomes on level 1 and level 2 oncoplastic procedures using BREAST-Q®. J Cancer Res Clin Oncol 149, 3229–3241 (2023). https://doi.org/10.1007/s00432-022-04228-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04228-2