Abstract
Purpose
Identifying patients at high risk of immune-related adverse events (irAEs) that impede the achievement of durable efficacy of programmed cell death 1 (PD-1)/programmed death ligand 1 (PD-L1) blockade therapy is important in improving their management. Identification of a novel predictive factor of therapeutic benefit is also important in improving patient selection for treatment with PD-1/PD-L1 inhibitors. Further determinants driving response and linking with irAEs are urgently required.
Methods
To address these unmet needs in the field, we explored whether 27 soluble checkpoint proteins and immunomodulatory proteins in serum at the therapy baseline and after week 3 were associated with irAE onset and therapeutic efficacy using MILLIPLEX Human Immuno-Oncology Checkpoint Protein Panel assays in a prospective, multicenter cohort of 81 patients with non-small cell lung cancer (NSCLC) receiving atezolizumab monotherapy.
Results
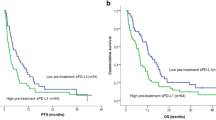
By competing-risks regression analysis, we identified that high levels of B cell-activating factor (BAFF) at baseline were a significant and strong risk factor of irAEs (hazard ratio, 5.61; 95% confidence interval, 2.43–12.96; P < 0.0001). We also identified that increased inducible T cell co-stimulator (ICOS) during the first therapeutic cycle was an independent factor associated with prolonged progression-free survival and overall survival.
Conclusion
These findings are in keeping with the reported mechanistic basis of these molecules and may provide potential guidance for clinical decision-making to improve patient care. Further validation studies are warranted.
Trial registration UMIN000035616 (January 28, 2019)


Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BAFF:
-
B cell-activating factor
- BTLA:
-
B and T lymphocyte attenuator
- CD40L:
-
CD40 ligand
- CI:
-
Confidence interval
- CTLA-4:
-
Cytotoxic T lymphocyte-associated protein 4
- DNAM-1:
-
DNAX accessory molecule 1
- FGL1:
-
Fibrinogen-like protein 1
- GITR:
-
Glucocorticoid-induced TNFR-related protein
- GITRL:
-
GITR ligand
- HR:
-
Hazard ratio
- HVEM:
-
Herpesvirus entry mediator
- ICI:
-
Immune-checkpoint inhibitor
- ICOS:
-
Inducible T cell co-stimulator
- ICOSL:
-
ICOS ligand
- irAE:
-
Immune-related adverse event
- LAG-3:
-
Lymphocyte-activation gene 3
- LIPI:
-
Lung immune prognostic index
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- PD-1:
-
Programmed cell death 1
- PD-L1:
-
Programmed death ligand 1
- PD-L2:
-
Programmed death ligand 2
- PFS:
-
Progression-free survival
- PVR:
-
Poliovirus receptor cell adhesion molecule
- TIM-3:
-
T cell immunoglobulin and mucin-domain containing-3
- TLR-2:
-
Toll-like receptor 2
- 4-1BBL:
-
4-1BB ligand
References
Abu-Sbeih H, Faleck DM, Ricciuti B et al (2020) Immune checkpoint inhibitor therapy in patients with preexisting inflammatory bowel disease. J Clin Oncol 38:576–583
Akbari O, Freeman GJ, Meyer EH et al (2002) Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med 8:1024–1032
Amatore F, Gorvel L, Olive D (2020) Role of inducible co-stimulator (ICOS) in cancer immunotherapy. Expert Opin Biol Ther 20:141–150
Antonia SJ, Villegas A, Daniel D et al (2017) Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 377:1919–1929
Bar N, Costa F, Das R et al (2020) Differential effects of PD-L1 versus PD-1 blockade on myeloid inflammation in human cancer. JCI Insight 5:e129353
Bomze D, Hasan Ali O, Bate A, Flatz L (2019) Association between immune-related adverse events during anti-PD-1 therapy and tumor mutational burden. JAMA Oncol 5:1633–1635
Cabrita R, Lauss M, Sanna A et al (2020) Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577:561–565
Cascone T, William WN Jr, Weissferdt A et al (2021) Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med 27:504–514
Chen J, Wang J, Xu H (2021) Comparison of atezolizumab, durvalumab, pembrolizumab, and nivolumab as first-line treatment in patients with extensive-stage small cell lung cancer: a systematic review and network meta-analysis. Medicine 100:e25180
Das R, Bar N, Ferreira M et al (2018) Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J Clin Invest 128:715–720
Dubin K, Callahan MK, Ren B et al (2016) Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun 7:10391
Felip E, Altorki N, Zhou C et al (2021) Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 398:1344–1357
Giannicola R, D’Arrigo G, Botta C et al (2019) Early blood rise in auto-antibodies to nuclear and smooth muscle antigens is predictive of prolonged survival and autoimmunity in metastatic-non-small cell lung cancer patients treated with PD-1 immune-check point blockade by nivolumab. Mol Clin Oncol 11:81–90
Gong B, Kiyotani K, Sakata S et al (2019) Secreted PD-L1 variants mediate resistance to PD-L1 blockade therapy in non-small cell lung cancer. J Exp Med 216:982–1000
Gorelik L, Gilbride K, Dobles M et al (2003) Normal B cell homeostasis requires B cell activation factor production by radiation-resistant cells. J Exp Med 198:937–945
Gorgulho J, Roderburg C, Heymann F et al (2021) Serum levels of soluble B and T lymphocyte attenuator predict overall survival in patients undergoing immune checkpoint inhibitor therapy for solid malignancies. Int J Cancer 149:1189–1198
Groom J, Kalled SL, Cutler AH et al (2002) Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren’s syndrome. J Clin Invest 109:59–68
Haslam A, Prasad V (2019) Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open 2:e192535
Havel JJ, Chowell D, Chan TA (2019) The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer 19:133–150
Helmink BA, Reddy SM, Gao J et al (2020) B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577:549–555
Hutloff A, Dittrich AM, Beier KC et al (1999) ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 397:263–266
Kanda Y (2013) Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transpl 48:452–458
Kreuzaler M, Rauch M, Salzer U et al (2012) Soluble BAFF levels inversely correlate with peripheral B cell numbers and the expression of BAFF receptors. J Immunol 188:497–503
Lavie F, Miceli-Richard C, Quillard J et al (2004) Expression of BAFF (BLyS) in T cells infiltrating labial salivary glands from patients with Sjogren’s syndrome. J Pathol 202:496–502
Lozano AX, Chaudhuri AA, Nene A et al (2022) T cell characteristics associated with toxicity to immune checkpoint blockade in patients with melanoma. Nat Med 28:353–362
Mackay F, Browning JL (2002) BAFF: a fundamental survival factor for B cells. Nat Rev Immunol 2:465–475
Mackay F, Woodcock SA, Lawton P et al (1999) Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med 190:1697–1710
Mezquita L, Auclin E, Ferrara R et al (2018) Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol 4:351–357
Moore PA, Belvedere O, Orr A et al (1999) BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science 285:260–263
Mukama T, Fortner RT, Katzke V et al (2022) Prospective evaluation of 92 serum protein biomarkers for early detection of ovarian cancer. Br J Cancer 126:1301–1309
Nardelli B, Belvedere O, Roschke V et al (2001) Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood 97:198–204
Petitprez F, de Reynies A, Keung EZ et al (2020) B cells are associated with survival and immunotherapy response in sarcoma. Nature 577:556–560
Rittmeyer A, Barlesi F, Waterkamp D et al (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389:255–265
Sarantopoulos S, Stevenson KE, Kim HT et al (2007) High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clin Cancer Res 13:6107–6114
Schneider P, MacKay F, Steiner V et al (1999) BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med 189:1747–1756
Solinas C, Gu-Trantien C, Willard-Gallo K (2020) The rationale behind targeting the ICOS-ICOS ligand costimulatory pathway in cancer immunotherapy. ESMO Open 5:e000544
Sullivan RJ, Weber JS (2021) Immune-related toxicities of checkpoint inhibitors: mechanisms and mitigation strategies. Nat Rev Drug Discov. https://doi.org/10.1038/s41573-021-00259-5
Suzuki Y, Karayama M, Uto T et al (2020) Assessment of immune-related interstitial lung disease in patients with NSCLC treated with immune checkpoint inhibitors: a multicenter prospective study. J Thorac Oncol 15:1317–1327
Xiao Z, Mayer AT, Nobashi TW, Gambhir SS (2020) ICOS Is an indicator of T-cell-mediated response to cancer immunotherapy. Cancer Res 80:3023–3032
Xu S, Shukuya T, Tamura J et al (2022) Heterogeneous outcomes of immune checkpoint inhibitor rechallenge in patients with NSCLC: a systematic review and meta-analysis. JTO Clin Res Rep 3:100309
Yanaba K, Asano Y, Noda S et al (2013) Increased production of soluble inducible costimulator in patients with diffuse cutaneous systemic sclerosis. Arch Dermatol Res 305:17–23
Yarchoan M, Ho WJ, Mohan A et al (2020) Effects of B cell-activating factor on tumor immunity. JCI Insight 5:e136417
Acknowledgements
The authors would like to thank the patients, their families, and all the investigators who participated in this study. We also thank H. Nikki March, Ph.D., from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
This work was supported by Chugai Pharmaceutical Co., Ltd. (Tokyo, Japan). The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Y.I.: Conceptualization, acquisition of data, data curation, formal analysis, investigation, methodology, project administration, resources, supervision, validation, visualization, writing—original draft, writing—review and editing. N.I.: Conceptualization, acquisition of data, data curation, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing—original draft, writing—review and editing. M.K, K.A., S.M., M.I., T.U., M.F., D.H., T.M., H.M., N.I., M.T., Y.K., H.Y., H.H., Y.S., K.F., N.E., T.F., T.S: Acquisition of data, data curation, supervision, validation, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Consent to participate
All patients provided written informed consent to participate in the study.
Ethics approval
This study was approved by the institutional review board at each site (Hamamatsu University School of Medicine, #18-164) and was conducted in accordance with the International Council for Harmonisation Good Clinical Practice guidelines and the Declaration of Helsinki. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines. This study was registered at the UMIN Clinical Trials Registry as UMIN000035616.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
432_2022_4193_MOESM1_ESM.tif
Supplementary file1 (TIF 961 KB) Supplementary Fig. S1. Subset analyses of BAFF according to EGFR genotype and prior treatment with immune checkpoint inhibitors (ICIs). a Serum BAFF values at therapeutic baseline in patients with EGFR wild-type (WT) tumors without prior ICI treatment (EGFR-WT, N = 56), EGFR-WT tumors with prior ICI treatment (ICI-rechallenged; N = 10), and EGFR-mutant tumors (N = 15). The Kruskal–Wallis test followed by adjustment using the method of Holm was applied. b Cumulative incidence curves for the onset of any-grade immune-related adverse events (irAEs) according to EGFR mutation status and history of prior ICI treatment. c and d Cumulative incidence curves for the onset of (c) any-grade irAEs and (d) pneumonitis according to serum BAFF levels in patients with EGFR-WT tumors without prior ICI treatment. e and f Cumulative incidence curves for the onset of (e) any-grade irAEs and (f) pneumonitis according to serum BAFF levels in patients with EGFR-mutant tumors.
432_2022_4193_MOESM2_ESM.tif
Supplementary file2 (TIF 937 KB) Supplementary Fig. S2. Prognostic impact of serum ICOS variation 3 weeks after atezolizumab initiation on progression-free survival (PFS) and overall survival (OS) in adenocarcinoma patients according to EGFR genotype. a. Kaplan–Meier curves of PFS according to serum ICOS variation in patients with EGFR-WT adenocarcinoma. b Kaplan–Meier curves of OS according to serum ICOS variation in patients with EGFR-WT adenocarcinoma. c Kaplan–Meier curves of PFS according to serum ICOS variation in patients with EGFR-mutant adenocarcinoma. d Kaplan–Meier curves of OS according to serum ICOS variation in patients with EGFR-mutant adenocarcinoma.
Rights and permissions
About this article
Cite this article
Inoue, Y., Inui, N., Karayama, M. et al. Serum immune modulators associated with immune-related toxicities and efficacy of atezolizumab in patients with non-small cell lung cancer. J Cancer Res Clin Oncol 149, 2963–2974 (2023). https://doi.org/10.1007/s00432-022-04193-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04193-w