Abstract
Objective
Afatinib is an oral, irreversible ErbB family blocker. It binds covalently to the kinase domains of epidermal growth factor (EGFR), HER2 and HER4, resulting in irreversible inhibition of tyrosine kinase autophosphorylation. Our trial compared the bioequivalence and safety between afatinib produced by Chia Tai Tianqing Pharmaceutical Group Co., Ltd. and Giotrif® produced by Boehringer Ingelheim.
Methods
Healthy Chinese subjects (N = 36) were randomly divided into two groups at a ratio of 1:1. There was a single dose per period of afatinib and Giotrif®. The washout was set as 14 days. Plasma drug concentrations of afatinib and Giotrif® were analyzed by liquid chromatography–tandem mass spectrometry (LC–MS/MS). Statistical analysis of major pharmacokinetic (PK) parameters was conducted to assess drug bioequivalence. In addition, we evaluated the safety of the drugs throughout the trial.
Results
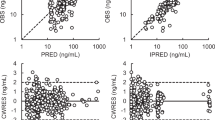
The geometric mean ratios (GMRs) of Cmax, AUC0−t, and AUC0−∞ for afatinib and Giotrif® were 102.80%, 101.83%, and 101.58%, respectively. The 90% confidence intervals (CIs) were all within 80%-125%, meeting the bioequivalence standards. In addition, both drugs showed a good safety profile during the trial.
Conclusion
This study showed that afatinib was bioequivalent to Giotrif® in healthy Chinese subjects with well safety.
Chinese Clinical trial registry
This trial is registered at the Chinese Clinical Trial website (http://www.chinadrugtrials.org.cn/index.html # CTR20171160).


Similar content being viewed by others
Date availability statement
We confirm that the figures and tables in the manuscript are original and not previously published. Data supporting the findings of this study may be obtained from the corresponding author upon reasonable request but remain subject to all applicable legal requirements to protect the confidentiality of the study participants’ personal information.
References
Al-Aidaroos AQ, Yuen HF, Guo K et al (2013) Metastasis-associated PRL-3 induces EGFR activation and addiction in cancer cells. J Clin Invest 123(8):3459–3471
Approved Drug Products with Therapeutic Equivalence Evaluations (2022). https://www.fda.gov/media/71474/download. Accessed 14 Jan 2022
Book (2022). ADPwTEEsEO. https://www.fda.gov/media/71474/download. Accessed 14 Jan 2022
El-Rayes BF, LoRusso PM (2004) Targeting the epidermal growth factor receptor. Br J Cancer 91(3):418–424
Freiwald M, Schmid U, Fleury A et al (2014) Population pharmacokinetics of afatinib, an irreversible ErbB family blocker, in patients with various solid tumors. Cancer Chemother Pharmacol 73(4):759–770
Gilotrif® (Afatinib) Tablets (2022). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/201292s014lbl.pdf. Accessed 15 Jan 2022
Giotrif (2016) Summary of productcharacteristics, version 24 May 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002280/WC500152392.pdf. Accessed 07 Jun 2016
Goh G, Schmid R, Guiver K et al (2016) Clonal evolutionary analysis during HER2 blockade in HER2-positive inflammatory breast cancer: a phase II open-label clinical trial of Afatinib +/- Vinorelbine. PLoS Med 13(12):e1002136
Hong S, Gao F, Fu S et al (2018) Concomitant genetic alterations with response to treatment and epidermal growth factor receptor tyrosine kinase inhibitors in patients with EGFR-mutant advanced non-small cell lung cancer. JAMA Oncol 4(5):739–742
Janne PA, Johnson BE (2006) Effect of epidermal growth factor receptor tyrosine kinase domain mutations on the outcome of patients with non-small cell lung cancer treated with epidermal growth factor receptor tyrosine kinase inhibitors. Clin Cancer Res 12(14 Pt 2):4416s–4420s
Janne PA, Boss DS, Camidge DR et al (2011) Phase I dose-escalation study of the pan-HER inhibitor, PF299804, in patients with advanced malignant solid tumors. Clin Cancer Res 17(5):1131–1139
Kharbanda A, Rajabi H, Jin C et al (2014) Targeting the oncogenic MUC1-C protein inhibits mutant EGFR-mediated signaling and survival in non-small cell lung cancer cells. Clin Cancer Res 20(21):5423–5434
Li D, Ambrogio L, Shimamura T et al (2008) BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 27(34):4702–4711
Lizotte PH, Hong RL, Luster TA et al (2018) A high-throughput immune-oncology screen identifies EGFR inhibitors as potent enhancers of antigen-specific cytotoxic t-lymphocyte tumor cell killing. Cancer Immunol Res 6(12):1511–1523
Maemondo M, Inoue A, Kobayashi K et al (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362(25):2380–2388
Marshall J, Hwang J, Eskens FA et al (2013) A Phase I, open-label, dose escalation study of afatinib, in a 3-week-on/1-week-off schedule in patients with advanced solid tumors. Invest New Drugs 31(2):399–408
Metro G, Crino L (2011) The LUX-Lung clinical trial program of afatinib for non-small-cell lung cancer. Expert Rev Anticancer Ther 11(5):673–682
Mok TS, Wu YL, Thongprasert S et al (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361(10):947–957
Moreira Sousa C, McGuire JR, Thion MS et al (2013) The Huntington disease protein accelerates breast tumour development and metastasis through ErbB2/HER2 signalling. EMBO Mol Med 5(2):309–325
O’Neill F, Madden SF, Clynes M et al (2013) A gene expression profile indicative of early stage HER2 targeted therapy response. Mol Cancer 1(12):69
Osborne CK, Neven P, Dirix LY et al (2011) Gefitinib or placebo in combination with tamoxifen in patients with hormone receptor-positive metastatic breast cancer: a randomized phase II study. Clin Cancer Res 17(5):1147–1159
Pirazzoli V, Ayeni D, Meador CB et al (2016) Afatinib plus cetuximab delays resistance compared to single-agent erlotinib or afatinib in mouse models of TKI-Naive EGFR L858R-Induced Lung Adenocarcinoma. Clin Cancer Res 22(2):426–435
Pollock NI, Wang L, Wallweber G et al (2015) Increased expression of HER2, HER3, and HER2:HER3 heterodimers in HPV-positive HNSCC using a novel proximity-based assay: implications for targeted therapies. Clin Cancer Res 21(20):4597–4606
Romanidou O, Landi L, Cappuzzo F et al (2016) Overcoming resistance to first/second generation epidermal growth factor receptor tyrosine kinase inhibitors and ALK inhibitors in oncogene-addicted advanced non-small cell lung cancer. Ther Adv Med Oncol 8(3):176–187
Siegel R, DeSantis C, Virgo K et al (2012) Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 62(4):220–241
Stopfer P, Marzin K, Narjes H et al (2012) Afatinib pharmacokinetics and metabolism after oral administration to healthy male volunteers. Cancer Chemother Pharmacol 69(4):1051–1061
Tian S, Simon I, Moreno V et al (2013) A combined oncogenic pathway signature of BRAF, KRAS and PI3KCA mutation improves colorectal cancer classification and cetuximab treatment prediction. Gut 62(4):540–549
Tzahar E, Waterman H, Chen X et al (1996) A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol 16(10):5276–5287
Wiebe S, Schnell D, Külzer R et al (2017) Influence of renal impairment on the pharmacokinetics of afatinib: an open-label, single-dose study. Eur J Drug Metab Pharmacokinet 42(3):461–469
Wind S, Schnell D, Ebner T et al (2017) Clinical Pharmacokinetics and Pharmacodynamics of Afatinib. Clin Pharmacokinet 56(3):235–250
Yamada Y, Tamura T, Yamamoto Y et al (2020) Treatment of patients with non-small-cell lung cancer with uncommon EGFR mutations in clinical practice. Anticancer Res 40(10):5757–5764
Yan X, Chen X, Liang H et al (2014) miR-143 and miR-145 synergistically regulate ERBB3 to suppress cell proliferation and invasion in breast cancer. Mol Cancer 24(13):220
Yu HA, Sima CS, Huang J et al (2013) Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol 8(3):346–351
Zhang J, Cao J, Li J et al (2014) A phase I study of AST1306, a novel irreversible EGFR and HER2 kinase inhibitor, in patients with advanced solid tumors. J Hematol Oncol 11(7):22
Acknowledgements
Chia Tai Tianqing Pharmaceutical Group Co., Ltd. provided the drugs (afatinib and Giotrif®). Thanks to all enrolled participants, investigators, and people who contributed to this study.
Funding
This work was supported by Chia Tai Tianqing Pharmaceutical Group Co.,Ltd, Nanjing, China. Funding number: phase I 2017-041. The founder helps pay for this study and has no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
GL and HY were involved in the conception and design of the trial. JX, YW, ZL, XL, XQ, ZQ, LS, MC, YW, XC, JY, DQ, LL and LZ, performed the research. YZ led the draft of the manuscript and participated in some statistics. QD, ZS and KX drafted the manuscript, figures and participated in some statistics. HY finally approved of the version. All authors agreed to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Conflict of interest
All data related to this study were interpreted by the trial staff with complete independence from the sponsor. Jinling Xue and Li Xue are employees of Chia Tai Tianqing Pharmaceutical Group Co.,Ltd; Guangwen Liu, Yanli Wang, Zhengzhi Liu, Xinyao Qu, Zhaojing Qu, Linlin Sun, Mingming Cao, Ying Wang, Lixiu, Zhang, Haimiao Yang are employees of Affiliated Hospital of Changchun University of Chinese Medicine. Qiaohuan Deng, Zhengjie Su and Kaibo Xu are graduate students of Affiliated Hospital of Changchun University of Chinese Medicine. Xuesong Chen, Jing Yu, Dongmei Qu, Lang Liu are employees of Ansiterui Medical Technology Consulting Co.,Ltd. Jilin, China. Yicheng Zhao is an employee of Puheng Technology Co., Ltd. The authors have no other relevant affiliations or financial involvement with any organization or entity with financial interest in or financial with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, G., Xue, J., Wang, Y. et al. A randomized, open-label, two-cycle, two-crossover phase I clinical trial comparing the bioequivalence and safety of afatinib and Giotrif® in healthy Chinese subjects. J Cancer Res Clin Oncol 149, 2585–2593 (2023). https://doi.org/10.1007/s00432-022-04148-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04148-1