Abstract
Purpose
For patients with advanced HCC, predictors of immunotherapy response are scarce, and the benefits of tyrosine kinase inhibitor (TKI) treatment after immunotherapy are unclear. We explored whether clinical features, such as target lesion response, immune-mediated toxicity, or subsequent TKI therapy predict immunotherapy response.
Methods
We retrospectively studied 77 patients with advanced HCC receiving immunotherapy. Patient characteristics and outcomes were assessed using various statistical methods, including the log-rank test and Kaplan–Meier methods. Cox proportional hazard modeling was used for multivariable survival analysis.
Results
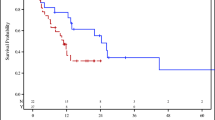
For all patients, median overall survival (mOS) was 13 months (95% CI 8–19), and median progression-free survival (mPFS) was 6 months (95% CI 4–10). Patients with partial response (PR) and stable disease (SD) compared to progressive disease (PD) had prolonged mPFS (27 vs. 5 vs. 1 month(s), p < 0.0001) and mOS (not met vs. 11 vs. 3 months, p < 0.0001). Patients with vs. without immune-mediated toxicities trended towards longer mPFS (9 vs. 4 months p = 0.133) and mOS (17 vs. 9 months; p = 0.095). Patients who did vs. did not receive a tyrosine kinase inhibitor (TKI) after immunotherapy had a significantly improved mOS (19 vs. 5 months, p = 0.0024)). Based on multivariate modeling, the hazard ratio (HR) of overall survival (OS) of patients receiving TKI vs. no TKI was 0.412 (p = 0.0043).
Conclusion
We show that disease control predicts prolonged mOS and mPFS. Furthermore, TKI therapy administered after immunotherapy predicts prolonged mOS in patients with advanced HCC.





Similar content being viewed by others
Availability of data and materials
Not applicable.
Abbreviations
- TKI:
-
Tyrosine kinase inhibitor
- SD:
-
Stable disease
- PD:
-
Disease progression
- PR:
-
Partial response
- PFS:
-
Progression-free survival
- mPFS:
-
Median progression-free survival
- OS:
-
Overall survival
- mOS:
-
Median overall survival
- TSH:
-
Thyroid-stimulating hormone
- PD-L1:
-
Programmed death-ligand 1
- PD-1:
-
Programmed cell death protein 1
- VEGF:
-
Anti-vascular endothelial growth factor
References
Ao H, Xin Z, Jian Z (2021) Liquid biopsy to identify biomarkers for immunotherapy in hepatocellular carcinoma. Biomark Res 9(1):91. https://doi.org/10.1186/s40364-021-00348-y
Aoki T, Nishida N, Ueshima K et al (2021) Higher enhancement intrahepatic nodules on the hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI as a poor responsive marker of anti-PD-1/PD-L1 monotherapy for unresectable hepatocellular carcinoma. Liver Cancer 2021 10(6):615–628. https://doi.org/10.1159/000518048
Auvray M, Auclin E, Barthelemy P et al (2019) Second-line targeted therapies after nivolumab-ipilimumab failure in metastatic renal cell carcinoma. Eur J Cancer 108:33–40. https://doi.org/10.1016/j.ejca.2018.11.031 (Erratum in: Eur J Cancer 2019; 119:200-201)
Bettinger D, Schultheiß M, Knuppel E, Thimme R, Blum H, Spangenberg H (2012) Diarrhoea predicts a positive response to sorafenib in patients with advanced hepatocellular carcinoma. Hepatology 56:789. https://doi.org/10.1002/hep.25637
Chang WT, Lu SN, Rau KM, Huang CS, Lee KT (2018) Increased cumulative doses and appearance of hand-foot skin reaction prolonged progression free survival in sorafenib-treated advanced hepatocellular carcinoma patients. Kaohsiung J Med Sci 34(7):391–399. https://doi.org/10.1016/j.kjms.2018.03.006
Cheng A, Kang YK, Chen Z et al (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase iii randomised, double-blind, placebo-controlled trial. Lancet Oncol 10(1):25–34. https://doi.org/10.1016/s1470-2045(08;70285-7
Cho J, Paik Y, Lim H et al (2013) Clinical parameters predictive of outcomes in sorafenib-treated patients with advanced hepatocellular carcinoma. Liver Int 33:950–957. https://doi.org/10.1111/liv.12168
Cunningham D, Humblet Y, Siena S et al (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337–345. https://doi.org/10.1056/NEJMoa033025
Di Costanzo G, De Stefano G, Tortora R et al (2015) Sorafenib off-target effects predict outcomes in patients treated for hepatocellular carcinoma. Future Oncol 11:943–951. https://doi.org/10.2217/fon.14.291
Di Fiore F, Rigal O, Ménager C, Michel P, Pfister C (2011) Severe clinical toxicities are correlated with survival in patients with advanced renal cell carcinoma treated with sunitinib and sorafenib. Br J Cancer 105:1811–1813. https://doi.org/10.1038/bjc.2011.507
El-Khoueiry A, Sangro B, Yau T et al (2017) Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389(10088):2492–2502. https://doi.org/10.1016/s0140-6736(17)31046-2
Faje AT, Lawrence D, Flaherty K et al (2018) High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 124(18):3706–3714. https://doi.org/10.1002/cncr.31629
Ferlay J, Colombet M, Soerjomataram I et al (2018) Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 144(8):1941–1953. https://doi.org/10.1002/ijc.31937
Finn R, Ryoo B, Merle P et al (2019) Results of KEYNOTE-240: phase 3 study of pembrolizumab (pembro) vs best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC). J Clin Oncol 37(15):suppl.4004. https://doi.org/10.1200/jco.2019.37.15_suppl.4004
Finn R, Qin S, Ikeda M et al (2020) Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl Jo Med 382(20):1894–1905. https://doi.org/10.1056/nejmoa1915745
Fontein D, Seynaeve C, Hadji P et al (2013) Specific adverse events predict survival benefit in patients treated with tamoxifen or aromatase inhibitors: an international tamoxifen exemestane adjuvant multinational trial analysis. J Clin Oncol 31:2257–2264. https://doi.org/10.1200/JCO.2012.45.3068
Germano D, Daniele B (2014) Systemic therapy of hepatocellular carcinoma: current status and future perspectives. World J Gastroenterol 20(12):3087–3099. https://doi.org/10.3748/wjg.v20.i12.3087
Goodwin R, Ding K, Seymour L et al (2010) Treatment-emergent hypertension and outcomes in patients with advanced non-small-cell lung cancer receiving chemotherapy with or without the vascular endothelial growth factor receptor inhibitor cediranib: NCIC clinical trials group study BR24. Ann Oncol 21:2220–2226. https://doi.org/10.1093/annonc/mdq221
Hamnvik O, Choueiri T, Turchin A et al (2015) Clinical risk factors for the development of hypertension in patients treated with inhibitors of the VEGF signaling pathway. Cancer 121:311–319. https://doi.org/10.1002/cncr.28972
Hiraoka A, Kumada T, Atsukawa M, Real-life Practice Experts for HCC (RELPEC) Study Group; HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan) et al (2019) Important clinical factors in sequential therapy including lenvatinib against unresectable hepatocellular carcinoma. Oncology 97(5):277–285. https://doi.org/10.1159/000501281 (Epub 2019 Jul 15)
Howell J, Pinato DJ, Ramaswami R et al (2017) On-target sorafenib toxicity predicts improved survival in hepatocellular carcinoma: a multi-centre, prospective study. Aliment Pharmacol Ther 45(8):1146–1155. https://doi.org/10.1111/apt.13977
Ikeda M, Mitsunaga S, Ohno I et al (2015) Systemic chemotherapy for advanced hepatocellular carcinoma: past, present, and future. Diseases 3(4):360–381. https://doi.org/10.3390/diseases3040360 (Published 2015 Dec 1)
Kole C, Charalampakis N, Tsakatikas S et al (2020) Immunotherapy for hepatocellular carcinoma: a 2021 update. Cancers (basel) 12(10):2859. https://doi.org/10.3390/cancers12102859
Koschny R, Gotthardt D, Koehler C, Jaeger D (2013) Diarrhoea is a positive outcome predictor for sorafenib treatment of advanced hepatocellular carcinoma. Oncology 84:6–13. https://doi.org/10.1159/000342425
Kudo M, Finn RS, Qin S et al (2018) Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391(10126):1163–1173. https://doi.org/10.1016/S0140-6736(18)30207-1
Llovet JM, Ricci S, Mazzaferro V et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–390. https://doi.org/10.1056/NEJMoa0708857
Morita M, Nishida N, Sakai K et al (2021) Immunological microenvironment predicts the survival of the patients with hepatocellular carcinoma treated with anti-PD-1 antibody. Liver Cancer 10(4):380–393. https://doi.org/10.1159/000516899
Osorio JC, Ni A, Chaft JE et al (2017) Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol 28(3):583–589. https://doi.org/10.1093/annonc/mdw640
Otsuka T, Eguchi Y, Kawazoe S, Yanagita K, Ario K, Kitahara K (2012) Skin toxicities and survival in advanced hepatocellular carcinoma patients treated with sorafenib. Hepatol Res 42:879–886. https://doi.org/10.1111/j.1872-034X.2012.00991
Shah AY, Kotecha RR, Lemke EA et al (2019) Outcomes of patients with metastatic clear-cell renal cell carcinoma treated with second-line VEGFR-TKI after first-line immune checkpoint inhibitors. Eur J Cancer 114:67–75. https://doi.org/10.1016/j.ejca.2019.04.003
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70(1):7–30. https://doi.org/10.3322/caac.21590
Teulings HE, Limpens J, Jansen SN et al (2015) Vitiligo-like depigmentation in patients with stage III–IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol 33(7):773–781. https://doi.org/10.1200/JCO.2014.57.4756
Vincenzi B, Santini D, Russo A, Addeo R (2010) Early skin toxicity as a predictive factor for tumour control in hepatocellular carcinoma patients treated with sorafenib. Oncologist 15:85–92. https://doi.org/10.1634/theoncologist.2009-0143
Wang Y, Abu-Sbeih H, Mao E et al (2018) Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: retrospective review at MD Anderson. J Immunother Cancer 6(1):37. https://doi.org/10.1186/s40425-018-0346-6
Yau T, Kang YK, Kim TY et al (2019a) Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (patients) with advanced hepatocellular carcinoma (aHCC): results from CheckMate 040. J Clin Oncol 37(15):suppl.4012. https://doi.org/10.1200/JCO.2019.37.15_suppl.4012
Yau T, Park JW, Finn RS et al (2019b) CheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (patients) with advanced hepatocellular carcinoma (AHCC). Ann Oncol 30:v874–v875. https://doi.org/10.1093/annonc/mdz394.029
Yeo W, Mok TS, Zee B et al (2005) A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst 97(20):1532–1538. https://doi.org/10.1093/jnci/dji315Zhu
Zhu A, Knox J, Kudo M et al (2017) KEYNOTE-224: phase II study of pembrolizumab in patients with previously treated advanced hepatocellular carcinoma. J Clin Oncol 35(4):suppl.TSP504. https://doi.org/10.1200/jco.2017.35.4_suppl.tps504
Funding
This work was supported by The Ruesch Center for the Cure of Gastrointestinal Cancers.
Author information
Authors and Affiliations
Contributions
All authors contributed to the research described in this manuscript. Drs. SA, TR, PP, MLH, and ARH were involved with the composition of the manuscript. XG and HW were involved in figure and table generation for the manuscript. All authors viewed and approved the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
Samantha Armstrong declares that she has no conflict of interest. Tina Roy declares that she has no conflict of interest. Bhavana Singh declares that she has no conflict of interest. Monika Kulasekaran declares that she has no conflict of interest. Fatima Shaukat declares that she has no conflict of interest. Xue Geng declares that she has no conflict of interest. Hongkun Wang declares that she has no conflict of interest. Petra Prins declares that she has no conflict of interest. Reena C. Jha declares that she has no conflict of interest. Marion L. Hartley, PhD declares that she has no conflict of interest. Aiwu Ruth He declares that she has no conflict of interest.
Code availability
Not applicable.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Armstrong, S., Roy, T., Singh, B. et al. TKIs beyond immunotherapy predict improved survival in advanced HCC. J Cancer Res Clin Oncol 149, 2559–2574 (2023). https://doi.org/10.1007/s00432-022-04115-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04115-w