Abstract
Purpose
To investigate the effectiveness and safety of the combination of sorafenib and drug-eluting bead transarterial chemoembolization (DEB-TACE) in the treatment of early intrahepatic stage-progressed advanced hepatocellular carcinoma (ISPA-HCC).
Methods
This study was approved by the ethics committees of six tertiary medical centers in China. Between October 2017 and October 2020, 213 patients with advanced HCC received either sorafenib combined with on-demand DEB-TACE (DTS group, n = 103) or sorafenib monotherapy (S group, n = 110). Overall survival (OS), time to progression (TTP), local tumor response, and adverse events (AEs) were compared between the two groups.
Results
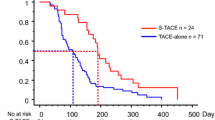
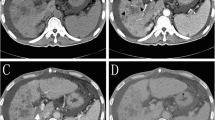
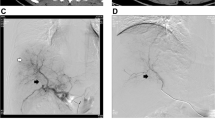
The incidences of nause/vomiting, abdonimal pain, hyperbilirubinemia, fever and ALT/AST increasing were higher in the DTS group. The post-treatment partial response, objective response, and disease control rates were significantly higher in the DTS group than in the S group (51.5% vs. 23.6%; 56.3% vs. 25.5%; 77.7% vs. 56.4%, respectively). The median OS was significantly longer in the DTS group than in the S group [16.3 vs. 10.0 months; hazard ratio (HR) = 0.43; P < 0.001], as was the TTP (6.7 vs. 4.3 months; HR = 0.60; P = 0.001). In the DTS group, patients who received ≥ 2 sessions of DEB-TACE benefited more than those who received two sessions of DEB-TACE. Multivariate analysis revealed that the α-fetoprotein level and treatment allocation were independent predictors of OS and TTP.
Conclusion
The combination of sorafenib and DEB-TACE is safe and effective for the treatment of early ISPA-HCC.




Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Brown DB, Nikolic B, Covey AM et al (2012) Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol 23:287–294. https://doi.org/10.1016/j.jvir.2011.11.029
Bruix J, Sherman M (2005) Practice guidelines committee, american association for the study of liver diseases management of hepatocellular carcinoma. Hepatology 42:1208–1236. https://doi.org/10.1002/hep.20933
European Association for the Study of the Liver (2018) EASL clinical practice guidelines easl clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 69:182–236. https://doi.org/10.1016/j.jhep.2018.03.019
Finn RS, Qin SK, Ikeda M et al (2020) Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 382:1894–1905. https://doi.org/10.1056/NEJMoa1915745
Finn RS, Qin SK, Ikeda M et al (2021) IMbrave150: updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) plus bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J Clin Oncol 39(3). https://doi.org/10.1200/JCO.2021.39.3_suppl.267
Hatooka M, Kawaoka T, Aikata H et al (2016) Comparison of outcome of hepatic arterial infusion chemotherapy and sorafenib in patients with hepatocellular carcinoma refractory to transcatheter arterial chemoembolization. Anticancer Res 36:3523–3529
Hiraoka A, Kumada T, Kudo M et al (2017) Albumin-bilirubin (ALBI) grade as part of the evidence-based clinical practice guideline for HCC of the Japan society of hepatology: a comparison with the liver damage and child-pugh classifications. Liver Cancer 6:204–215. https://doi.org/10.1159/000452846
Ikeda M, Mitsunaga S, Shimizu S et al (2014) Efficacy of sorafenib in patients with hepatocellular carcinoma refractory to transcatheter arterial chemoembolization. J Gastroenterol 49:932–940. https://doi.org/10.1007/s00535-013-0853-7
Johnson PJ, Kalayci C, Dobbs N et al (1991) Pharmacokinetics and toxicity of intraarterial adriamycin for hepatocellular carcinoma: effect of coadministration of lipiodol. J Hepatol 13:120–127. https://doi.org/10.1016/0168-8278(91)90873-a
Kim HY, Park JW, Joo J et al (2012) Severity and timing of progression predict refractoriness to transarterial chemoembolization in hepatocellular carcinoma. J Gastroenterol Hepatol 27:1051–1056. https://doi.org/10.1111/j.1440-1746.2011.06963.x
Kudo M, Matsui O, Izumi N et al (2014) JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver Cancer 3:58–68. https://doi.org/10.1159/000343875
Kudo M, Arizumi T, Ueshima K, Sakurai T, Kitano M, Nishida N (2015) Subclassification of BCLC B stage hepatocellular carcinoma and treatment strategies: proposal of modified bolondi’s subclassification (kinki criteria). Dig Dis 33:751–758. https://doi.org/10.1159/000439290
Kudo M, Paoul J, Lee H et al (2018) Deterioration of liver function after transarterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): an exploratory analysis of OPTIMIS-an international observational study assessing the use of sorafenib after TACE. J Clin Oncol. https://doi.org/10.1200/JCO.2018.36.4_suppl.368
Kudo M, Ueshima K, Ikeda M et al (2020) Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut 69:1492–1501. https://doi.org/10.1136/gutjnl-2019-318934
Lammer J, Malagari K, Vogl T et al (2010) Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the precision V study. Cardiovasc Intervent Radiol 33:41–52. https://doi.org/10.1007/s00270-009-9711-7
Lee JK, Chung YH, Song BC et al (2002) Recurrences of hepatocellular carcinoma following initial remission by transcatheter arterial chemoembolization. J Gastroenterol Hepatol 17:52–58. https://doi.org/10.1046/j.1440-1746.2002.02664.x
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30:52–60. https://doi.org/10.1055/s-0030-1247132
Lencioni R, de Baere T, Burrel M et al (2012) Transcatheter treatment of hepatocellular carcinoma with doxorubicin-loaded DC bead (DEBDOX): technical recommendations. Cardiovasc Interv Radiol 35:980–985. https://doi.org/10.1007/s00270-011-0287-7
Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF (2016a) Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology 64:106–116. https://doi.org/10.1002/hep.28453
Lencioni R, Llovet JM, Han G et al (2016b) Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J Hepatol 64:1090–1098. https://doi.org/10.1016/j.jhep.2016.01.012
Liu J, Xu J, Zhang W et al (2020) Safety and efficacy of drug-eluting bead transarterial chemoembolization combined with apatinib in patients with advanced hepatocellular carcinoma. Acad Radiol 27:704–709. https://doi.org/10.1016/j.acra.2019.07.003
Meyer T, Fox R, Ma YT et al (2017) Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol 2:565–575. https://doi.org/10.1016/S2468-1253(17)30156-5
National Cancer Institute, National Institutes of Health (2017) Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 Published: November 27, 2017 U.S. Department Of Health And Human Services. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf. Accessed 31 Aug 2021.
Oh D, Shin SW, Park HC, Cho SK, Lim DH, Paik SW (2015) Changes in arterioportal shunts in hepatocellular carcinoma patients with portal vein thrombosis who were treated with chemoembolization followed by radiotherapy. Cancer Res Treat 47:251–258. https://doi.org/10.4143/crt.2014.011
Park JW, Koh YH, Kim HB et al (2012) Phase II study of concurrent transarterial chemoembolization and sorafenib in patients with unresectable hepatocellular carcinoma. J Hepatol 56:1336–1342. https://doi.org/10.1016/j.jhep.2012.01.006
Park JW, Kim YJ, Kim DY et al (2019) Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: the phase III STAH trial. J Hepatol 70:684–691. https://doi.org/10.1016/j.jhep.2018.11.029
Pawlik TM, Reyes DK, Cosgrove D, Kamel IR, Bhagat N, Geschwind JF (2011) Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J Clin Oncol 29:3960–3967. https://doi.org/10.1200/JCO.2011.37.1021
Peck-Radosavljevic M, Kudo M, Lee HC et al (2018) Real-world data in patients with hepatocellular carcinoma treated with transarterial chemoembolization: the second interim analysis of OPTIMIS. J Hepatol 68:S202–S202. https://doi.org/10.1016/S0168-8278(18)30614-7
Peck-Radosavljevic M, Lee H, Kudo M (2019) Practice patterns and outcomes of transarterial chemoembolization in patients with hepatocellular carcinoma who were ineligible and eligible for transarterial chemoembolization at inclusion: global OPTIMIS exploratory analysis. J Hepatol 70:E616–E616. https://doi.org/10.1016/S0618-8278(19)31229-0
Seki A, Hori S (2012) Switching the loaded agent from epirubicin to cisplatin: salvage transcatheter arterial chemoembolization with drug-eluting microspheres for unresectable hepatocellular carcinoma. Cardiovasc Interv Radiol 35:555–562. https://doi.org/10.1007/s00270-011-0176-0
Sieghart W, Hucke F, Peck-Radosavljevic M (2015) Transarterial chemoembolization: modalities, indication, and patient selection. J Hepatol 62:1187–1195. https://doi.org/10.1016/j.jhep.2015.02.010
Song DS, Choi JY, Yoo SH et al (2013) DC bead transarterial chemoembolization is effective in hepatocellular carcinoma refractory to conventional transarteral chemoembolization: a pilot study. Gut Liver 7:89–95. https://doi.org/10.5009/gnl.2013.7.1.89
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Vogl TJ, Naguib NNN, Nour-Eldin NE et al (2009) Review on transarterial chemoembolization in hepatocellular carcinoma: palliative, combined, neoadjuvant, bridging, and symptomatic indications. Eur J Radiol 72:505–516. https://doi.org/10.1016/j.ejrad.2008.08.007
Vogl TJ, Lammer J, Lencioni R et al (2011) Liver, gastrointestinal, and cardiac toxicity in intermediate hepatocellular carcinoma treated with precision TACE with drug-eluting beads: results from the precision V randomized trial. AJR Am J Roentgenol 197:W562–W570. https://doi.org/10.2214/AJR.10.4379
Wu FX, Chen J, Bai T et al (2017) The safety and efficacy of transarterial chemoembolization combined with sorafenib and sorafenib mono-therapy in patients with BCLC stage B/C hepatocellular carcinoma. BMC Cancer 17:645. https://doi.org/10.1186/s12885-017-3545-5
Xiao YD, Ma C, Zhang ZS, Liu J (2019) Safety and efficacy assessment of transarterial chemoembolization using drug-eluting beads in patients with hepatocellular carcinoma and arterioportal shunt: a single-center experience. Cancer Manag Res 11:1551–1557. https://doi.org/10.2147/CMAR.S193948
Zhu K, Chen J, Lai L et al (2014) Hepatocellular carcinoma with portal vein tumor thrombus: treatment with transarterial chemoembolization combined with sorafenib—a retrospective controlled study. Radiology 272:284–293. https://doi.org/10.1148/radiol.14131946
Funding
This research was supported by the National Natural Science Foundation of China (Grant number: 81971719), the Natural Science Foundation of Guangdong Province (Grant number: 2021A1515010548) and Guangdong Medical Science and Technology Research Fund (Grant number: A2020081).
Author information
Authors and Affiliations
Contributions
Conceptualization: JL and WF. Data curation: BZ, SY, ML, HF, LQ, FLi, GY, YW, XZ, HW, and MX. Formal analysis: BZ, XZ and SY. Funding acquisition: JL, WF and YW. Investigation: ML, HF, LQ, FL, GY, YW, XZ, HW, and MX. Methodology: WF, BZ and XZ. Project administration: JL. Resources: JL, WF, ML, HF, LQ, FL, and GY. Supervision: JL. Visualization: JL and WF. Writing–original draft: BZ. Writing–review and editing: WF, JL, BZ and XZ.
Corresponding author
Ethics declarations
Conflict of interest
Authors declared no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fan, W., Zhu, B., Zheng, X. et al. Sorafenib plus drug-eluting bead transarterial chemoembolization for early intrahepatic stage-progressed advanced hepatocellular carcinoma refractory to conventional transarterial chemoembolization. J Cancer Res Clin Oncol 149, 1873–1882 (2023). https://doi.org/10.1007/s00432-022-04107-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04107-w