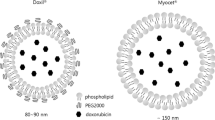
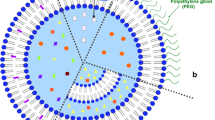
Abstract
Purpose
Lung cancer is the leading cause of cancer mortality worldwide. To improve the therapeutic outcomes, drug delivery systems, and particularly liposomes, have been widely investigated. Therefore, this review analyzed systematically the literature to inquire about the safety and efficacy of liposomal formulations in lung cancer treatment.
Methods
Three electronic databases (PubMed, Web of Science and Cochrane CENTRAL) were systematically searched until May 2020. Clinical trials containing information about the effects of liposomal formulations in lung cancer patients were considered eligible.
Results
Twenty two selected studies present different treatment options for both small and non-small-cell lung cancers. After compiling and analyzing all the published information, we verified that combination of liposomal cisplatin and paclitaxel led to a statistically significant improvement of the evaluated outcomes. Moreover, tecemotide, a liposome-based immunotherapy, demonstrated lower toxicity compared to control groups. Evidences that other subgroups could benefit from this formulation were also provided.
Conclusion
This systematic review (registration number CRD42021246587) demonstrates that liposomal formulations are promising alternatives to overcome limitations of traditional cancer therapy. However, larger, longer, randomized and double-blinded clinical trials, selecting their patients’ cohort considering more responsive subgroups would be beneficial to strengthen the scientific and clinical evidence of the results herein reported.

Similar content being viewed by others
References
Boulikas T (2009) Clinical overview on lipoplatin: a successful liposomal formulation of cisplatin. Expert Opin Investig Drugs 18:1197–1218
Bulbake U, Doppalapudi S, Kommineni N, Khan W (2017) Liposomal formulations in clinical use: an updated review. Pharmaceutics 9:12
Butts C, Maksymiuk A, Goss G, Soulières D, Marshall E et al (2011) Updated survival analysis in patients with stage IIIB or IV non-small-cell lung cancer receiving BLP25 liposome vaccine (L-BLP25): phase IIB randomized, multicenter, open-label trial. J Cancer Res Clin Oncol 137:1337–1342
Butts C, Murray N, Maksymiuk A, Goss G, Marshall E et al (2005) Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol 23:6674–6681
Butts C, Murray RN, Smith CJ, Ellis PM, Jasas K et al (2010) A multicenter open-label study to assess the safety of a new formulation of BLP25 liposome vaccine in patients with unresectable stage III non-small-cell lung cancer. Clin Lung Cancer 11:391–395
Butts C, Socinski MA, Mitchell PL, Thatcher N, Havel L et al (2014) Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol 15:59–68
CASP (2018) Critical appraisal skills programme: randomised controlled trial 2018
Chen G, Sheng L, Du X (2018) Efficacy and safety of liposome-paclitaxel and carboplatin based concurrent chemoradiotherapy for locally advanced lung squamous cell carcinoma. Cancer Chemother Pharmacol 82:505–510
Giodini L, Re FL, Campagnol D, Marangon E, Posocco B et al (2017) Nanocarriers in cancer clinical practice: a pharmacokinetic issue. Nanomedicine 13:583–599
Institute NC (2020) Common terminology criteria for adverse events v6.0 (CTCAE)
Katakami N, Hida T, Nokihara H, Imamura F, Sakai H et al (2017) Phase I/II study of tecemotide as immunotherapy in Japanese patients with unresectable stage III non-small cell lung cancer. Lung Cancer 105:23–30
Lawrie TA, Bryant A, Cameron A, Gray E, Morrison J (2013) Pegylated liposomal doxorubicin for relapsed epithelial ovarian cancer. Cochrane Database Syst Rev 2013:Cd006910
Leighl NB, Burkes RL, Dancey JE, Lopez PG, Higgins BP et al (2003) A phase I study of pegylated liposomal doxorubicin hydrochloride (Caelyx) in combination with cyclophosphamide and vincristine as second-line treatment of patients with small-cell lung cancer. Clin Lung Cancer 5:107–112
Leighl NB, Goss GD, Lopez PG, Burkes RL, Dancey JE et al (2006) Phase II study of pegylated liposomal doxorubicin HCl (Caelyx) in combination with cyclophosphamide and vincristine as second-line treatment of patients with small cell lung cancer. Lung Cancer 52:327–332
Lu B, Sun L, Yan X, Ai Z, Xu J (2015) Intratumoral chemotherapy with paclitaxel liposome combined with systemic chemotherapy: a new method of neoadjuvant chemotherapy for stage III unresectable non-small cell lung cancer. Med Oncol 32:345
Lv WZ, Lin Z, Wang SY, Lv BJ, Wang ZH et al (2019) Phase II study of a Bi-weekly chemotherapy regimen of combined liposomal paclitaxel and nedaplatin for the treatment of advanced squamous cell lung cancer. Transl Oncol 12:656–660
Menon JU, Kuriakose A, Iyer R, Hernandez E, Gandee L et al (2017) Dual-drug containing core-shell nanoparticles for lung cancer therapy. Sci Rep 7:13249
Mitchell P, Thatcher N, Socinski MA, Wasilewska-Tesluk E, Horwood K et al (2015) Tecemotide in unresectable stage III non-small-cell lung cancer in the phase III START study: updated overall survival and biomarker analyses. Ann Oncol 26:1134–1142
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Mylonakis N, Athanasiou A, Ziras N, Angel J, Rapti A et al (2010) Phase II study of liposomal cisplatin (Lipoplatin) plus gemcitabine versus cisplatin plus gemcitabine as first line treatment in inoperable (stage IIIB/IV) non-small cell lung cancer. Lung Cancer 68:240–247
Novello S, Barlesi F, Califano R, Cufer T, Ekman S et al (2016) Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol 27:v1–v27
Numico G, Castiglione F, Granetto C, Garrone O, Mariani G et al (2002) Single-agent pegylated liposomal doxorubicin (Caelix) in chemotherapy pretreated non-small cell lung cancer patients: a pilot trial. Lung Cancer 35:59–64
Palmer M, Parker J, Modi S, Butts C, Smylie M et al (2001) Phase I study of the BLP25 (MUC1 peptide) liposomal vaccine for active specific immunotherapy in stage IIIB/IV non-small-cell lung cancer. Clin Lung Cancer 3:49–57 (Discussion 58)
Patlakas G, Bouros D, Tsantekidou-Pozova S, Koukourakis MI (2005) Triplet chemotherapy with docetaxel, gemcitabine and liposomal doxorubicin, supported with subcutaneous amifostine and hemopoietic growth factors, in advanced non-small cell lung cancer. Anticancer Res 25:1427–1431
Ravaioli A, Papi M, Pasquini E, Marangolo M, Rudnas B et al (2009) Lipoplatin monotherapy: a phase II trial of second-line treatment of metastatic non-small-cell lung cancer. J Chemother 21:86–90
Sharma B, Crist RM, Adiseshaiah PP (2017) Nanotechnology as a delivery tool for precision cancer therapies. AAPS J 19:1632–1642
Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y et al (2003) Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 73:712–716
Stathopoulos GP, Antoniou D, Dimitroulis J, Michalopoulou P, Bastas A et al (2010) Liposomal cisplatin combined with paclitaxel versus cisplatin and paclitaxel in non-small-cell lung cancer: a randomized phase III multicenter trial. Ann Oncol 21:2227–2232
Stathopoulos GP, Antoniou D, Dimitroulis J, Stathopoulos J, Marosis K et al (2011) Comparison of liposomal cisplatin versus cisplatin in non-squamous cell non-small-cell lung cancer. Cancer Chemother Pharmacol 68:945–950
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
Villaruz LC, Socinski MA (2013) The clinical viewpoint: definitions, limitations of RECIST, practical considerations of measurement. Clin Cancer Res 19:2629–2636
Vokes EE, Gordon GS, Mauer AM, Rudin CM, Krauss SA et al (2000) A phase I study of STEALTH cisplatin (SPI-77) and vinorelbine in patients with advanced non small-cell lung cancer. Clin Lung Cancer 2:128–132
Wang X, Zhou J, Wang Y, Zhu Z, Lu Y et al (2010) A phase I clinical and pharmacokinetic study of paclitaxel liposome infused in non-small cell lung cancer patients with malignant pleural effusions. Eur J Cancer 46:1474–1480
Weiss RB, Donehower RC, Wiernik PH, Ohnuma T, Gralla RJ et al (1990) Hypersensitivity reactions from taxol. J Clin Oncol 8:1263–1268
White SC, Lorigan P, Margison GP, Margison JM, Martin F et al (2006) Phase II study of SPI-77 (sterically stabilised liposomal cisplatin) in advanced non-small-cell lung cancer. Br J Cancer 95:822–828
Woodman C, Vundu G, George A, Wilson CM (2021) Applications and strategies in nanodiagnosis and nanotherapy in lung cancer. Semin Cancer Biol 69:349–364
Wurz GT, Kao C-J, Wolf M, DeGregorio MW (2014) Tecemotide: an antigen-specific cancer immunotherapy. Hum Vaccin Immunother 10:3383–3393
Xenidis N, Vardakis N, Varthalitis I, Giassas S, Kontopodis E et al (2011) Α multicenter phase II study of pegylated liposomal doxorubicin in combination with irinotecan as second-line treatment of patients with refractory small-cell lung cancer. Cancer Chemother Pharmacol 68:63–68
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Authors have no conflicts of interest.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Canão, F., Ferreira, H. & Neves, N.M. Liposomal formulations for lung cancer treatment in the last two decades: a systematic review. J Cancer Res Clin Oncol 148, 2375–2386 (2022). https://doi.org/10.1007/s00432-022-04079-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04079-x