Abstract
Purpose
Immune checkpoint inhibitors (ICIs) have prolonged the survival of patients with various carcinomas, including non-small cell lung cancer (NSCLC), and have caused a paradigm shift in cancer treatment. Although programmed death-ligand 1 (PD-L1) expression in tumor cells is a predictive marker of therapeutic efficacy, additional predictive markers are required. This study aimed to explore the role of immunological and nutritional parameters in the prediction of treatment response.
Methods
Patients diagnosed with NSCLC and treated with pembrolizumab were examined retrospectively. Body weight was measured 4–6 weeks before the start of the first treatment, immediately before treatment, and 4–6 weeks after the start of the first treatment. Progression-free survival (PFS) was defined as the time from the start of pembrolizumab treatment to the last follow-up date or until disease progression. Statistical analyses were performed to confirm the association between various factors and association between these factors and PFS.
Results
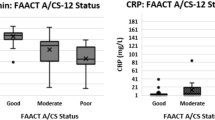
Thirty-eight patients with advanced NSCLC were included. We observed a significant association of weight loss and PD-L1 expression with PFS in the multivariate analysis. A significant correlation was found between the advanced lung cancer inflammation index and neutrophil-to-lymphocyte ratio. A weight loss of > 5% after the start of treatment was significantly associated with worse PFS.
Conclusions
Weight loss is an important negative prognostic factor in patients with NSCLC receiving immunotherapy. Weight maintenance may be important for good ICI treatment efficacy, and future interventions in cancer cachexia are expected to further enhance the treatment efficacy of ICIs.


Similar content being viewed by others
Availability of data
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aguilar EJ, Ricciuti B, Gainor JF, Kehl KL, Kravets S, Dahlberg S, Nishino M, Sholl LM, Adeni A, Subegdjo S, Khosrowjerdi S, Peterson RM, Digumarthy S, Liu C, Sauter J, Rizvi H, Arbour KC, Carter BW, Heymach JV, Altan M, Hellmann MD, Awad MM (2019) Outcomes to first-line pembrolizumab in patients with non-small-cell lung cancer and very high PD-L1 expression. Ann Oncol 30:1653–1659. https://doi.org/10.1093/annonc/mdz288
Amrane K, Geier M, Corre R, Léna H, Léveiller G, Gadby F, Lamy R, Bizec JL, Goarant E, Robinet G, Gouva S, Quere G, Abgral R, Schick U, Bernier C, Chouaid C, Descourt R (2020) First-line pembrolizumab for non-small cell lung cancer patients with PD-L1 ≥50% in a multicenter real-life cohort: the PEMBREIZH study. Cancer Med 7:2309–2316. https://doi.org/10.1002/cam4.2806
Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL, Kosteva JA, Ciunci CA, Gabriel PE, Thompson JC, Stonehouse-Lee S, Sherry VE, Gilbert E, Eaby-Sandy B, Mutale F, DiLullo G, Cohen RB, Vachani A, Langer CJ (2017) Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 106:1–7. https://doi.org/10.1016/j.lungcan.2017.01.013
Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI (2010) Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg 200:197–203. https://doi.org/10.1016/j.amjsurg.2009.08.041
Chan AW, Chan SL, Wong GL, Wong VW, Chong CC, Lai PB, Chan HL, To KF (2015) Prognostic nutritional index (PNI) predicts tumor recurrence of very early/early stage hepatocellular carcinoma after surgical resection. Ann Surg Oncol 22:4138–4148. https://doi.org/10.1245/s10434-015-4516-1
Cortellini A, Bersanelli M, Buti S et al (2019) A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer 7:57. https://doi.org/10.1186/s40425-019-0527-y
Cortellini A, Bozzetti F, Palumbo P et al (2020) Weighing the role of skeletal muscle mass and muscle density in cancer patients receiving PD-1/PD-L1 checkpoint inhibitors: a multicenter real-life study. Sci Rep 10:1456. https://doi.org/10.1038/s41598-020-58498-2
Derstine BA, Holcombe SA, Ross BE, Wang NC, Su GL, Wang SC (2018) Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci Rep 8:11369. https://doi.org/10.1038/s41598-018-29825-5
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 5:489–495. https://doi.org/10.1016/S1470-2045(10)70218-7
Fearon KC, Glass DJ, Guttridge DC (2012) Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab 16:153–166. https://doi.org/10.1016/j.cmet.2012.06.011
Gettinger S, Horn L, Jackman D, Spigel D, Antonia S, Hellmann M, Powderly J, Heist R, Sequist LV, Smith DC, Leming P, Geese WJ, Yoon D, Li A, Brahmer J (2018) Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: results from the CA209-003 study. J Clin Oncol 36:1675–1684. https://doi.org/10.1200/JCO.2017.77.0412
Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Hammad A, Tamai Y, Inagaki N, Uemoto S (2016) Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition 32:1200–1205. https://doi.org/10.1016/j.nut.2016.04.003
Haruki K, Shiba H, Shirai Y, Horiuchi T, Iwase R, Fujiwara Y, Furukawa K, Misawa T, Yanaga K (2016) The C-reactive protein to albumin ratio predicts long-term outcomes in patients with pancreatic cancer after pancreatic resection. World J Surg 40:2254–2260. https://doi.org/10.1007/s00268-016-3491-4
Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387:1540–1550. https://doi.org/10.1016/S0140-6736(15)01281-7
Jafri SH, Shi R, Mills G (2013) Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC Cancer 13:158. https://doi.org/10.1186/1471-2407-13-158
Johannet P, Sawyers A, Qian Y, Kozloff S, Gulati N, Donnelly D, Zhong J, Osman I (2020) Baseline prognostic nutritional index and changes in pretreatment body mass index associate with immunotherapy response in patients with advanced cancer. J Immunother Cancer 8:e001674. https://doi.org/10.1136/jitc-2020-001674
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458. https://doi.org/10.1038/bmt.2012.244
Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A (2011) Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg 98:268–274. https://doi.org/10.1002/bjs.7305
Katakami N, Uchino J, Yokoyama T, Naito T, Kondo M, Yamada K, Kitajima H, Yoshimori K, Sato K, Saito H, Aoe K, Tsuji T, Takiguchi Y, Takayama K, Komura N, Takiguchi T, Eguchi K (2018) Anamorelin (ONO-7643) for the treatment of patients with non-small cell lung cancer and cachexia: Results from a randomized, double-blind, placebo-controlled, multicenter study of Japanese patients (ONO-7643–04). Cancer 124:606–616. https://doi.org/10.1002/cncr.31128
Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Tanaka K, Fushiya N, Koike K, Nishino H, Matsushima M (2015) The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol 22:803–810. https://doi.org/10.1245/s10434-014-4048-0
Kyi C, Postow MA (2014) Checkpoint blocking antibodies in cancer immunotherapy. FEBS Lett 588:368–376. https://doi.org/10.1016/j.febslet.2013.10.015
Magri V, Gottfried T, Di Segni M, Urban D, Peled M, Daher S, Stoff R, Bar J, Onn A (2019) Correlation of body composition by computerized tomography and metabolic parameters with survival of nivolumab-treated lung cancer patients. Cancer Manag Res 11:8201–8207. https://doi.org/10.2147/CMAR.S210958
Martin L, Senesse P, Gioulbasanis I, Antoun S, Bozzetti F, Deans C, Strasser F, Thoresen L, Jagoe RT, Chasen M, Lundholm K, Bosaeus I, Fearon KH, Baracos VE (2015) Diagnostic criteria for the classification of cancer-associated weight loss. J Clin Oncol 33:90–99. https://doi.org/10.1200/JCO.2014.56.1894
Mathew M, Safyan RA, Shu CA (2017) PD-L1 as a biomarker in NSCLC: challenges and future directions. Ann Transl Med 5:375. https://doi.org/10.21037/atm.2017.08.04
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK, Bondarenko I, Kubota K, Lubiniecki GM, Zhang J, Kush D, Lopes G, KEYNOTE-042 Investigators (2019) Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 393(10183):1819–1830. https://doi.org/10.1016/S0140-6736(18)32409-7
Motomura T, Shirabe K, Mano Y, Muto J, Toshima T, Umemoto Y, Fukuhara T, Uchiyama H, Ikegami T, Yoshizumi T, Soejima Y, Maehara Y (2013) Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol 58:58–64. https://doi.org/10.1016/j.jhep.2012.08.017
Mouw KW, Goldberg MS, Konstantinopoulos PA, D’Andrea AD (2017) DNA damage and repair biomarkers of immunotherapy response. Cancer Discov 7:675–693. https://doi.org/10.1158/2159-8290.CD-17-0226
Naik GS, Waikar SS, Johnson AEW, Buchbinder EI, Haq R, Hodi FS, Schoenfeld JD, Ott PA (2019) Complex inter-relationship of body mass index, gender and serum creatinine on survival: exploring the obesity paradox in melanoma patients treated with checkpoint inhibition. J Immunother Cancer 7:89. https://doi.org/10.1186/s40425-019-0512-5
Nasser NJ, Gorenberg M, Agbarya A (2020) First line immunotherapy for non-small cell lung cancer. Pharmaceuticals (Basel) 13:373. https://doi.org/10.3390/ph13110373
Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR, KEYNOTE-024 Investigators (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823–1833. https://doi.org/10.1056/NEJMoa1606774
Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leal TA, Riess JW, Jensen E, Zhao B, Pietanza MC, Brahmer JR (2021) Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with Pd-L1 tumor proportion score ≥ 50. J Clin Oncol 39:2339–2349. https://doi.org/10.1200/JCO.21.00174
Ribas A, Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 359:1350–1355. https://doi.org/10.1126/science.aar4060
Roch B, Coffy A, Jean-Baptiste S, Palaysi E, Daures JP, Pujol JL, Bommart S (2020) Cachexia - sarcopenia as a determinant of disease control rate and survival in non-small lung cancer patients receiving immune-checkpoint inhibitors. Lung Cancer 143:19–26. https://doi.org/10.1016/j.lungcan.2020.03.003
Sha D, Jin Z, Budczies J, Kluck K, Stenzinger A, Sinicrope FA (2020) Tumor mutational burden as a predictive biomarker in solid tumors. Cancer Discov 10:1808–1825. https://doi.org/10.1158/2159-8290.CD-20-0522
Sharaiha RZ, Halazun KJ, Mirza F, Port JL, Lee PC, Neugut AI, Altorki NK, Abrams JA (2011) Elevated preoperative neutrophil:lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol 18:3362–3369. https://doi.org/10.1245/s10434-011-1754-8
Shibata M, Fukahori M, Kasamatsu E, Machii K, Hamauchi S (2020) A retrospective cohort study to investigate the incidence of cachexia during chemotherapy in patients with colorectal cancer. Adv Ther 37:5010–5022. https://doi.org/10.1007/s12325-020-01516-6
Shiroyama T, Suzuki H, Tamiya M, Tamiya A, Tanaka A, Okamoto N, Nakahama K, Taniguchi Y, Isa SI, Inoue T, Imamura F, Atagi S, Hirashima T (2018) Pretreatment advanced lung cancer inflammation index (ALI) for predicting early progression in nivolumab-treated patients with advanced non-small cell lung cancer. Cancer Med 7:13–20. https://doi.org/10.1002/cam4.1234
Takayama K, Atagi S, Imamura F, Tanaka H, Minato K, Harada T, Katakami N, Yokoyama T, Yoshimori K, Takiguchi Y, Hataji O, Takeda Y, Aoe K, Kim YH, Yokota S, Tabeta H, Tomii K, Ohashi Y, Eguchi K, Watanabe K (2016) Quality of life and survival survey of cancer cachexia in advanced non-small cell lung cancer patients-Japan nutrition and QOL survey in patients with advanced non-small cell lung cancer study. Support Care Cancer 24:3473–3480. https://doi.org/10.1007/s00520-016-3156-8
Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, Fearon KC (2016) Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol 17:519–531. https://doi.org/10.1016/S1470-2045(15)00558-6
Toiyama Y, Shimura T, Yasuda H, Fujikawa H, Okita Y, Kobayashi M, Ohi M, Yoshiyama S, Hiro J, Araki T, Inoue Y, Mohri Y, Kusunoki M (2016) Clinical burden of C-reactive protein/albumin ratio before curative surgery for patients with gastric cancer. Anticancer Res 36:6491–6498. https://doi.org/10.21873/anticanres.11248
Ubukata H, Motohashi G, Tabuchi T, Nagata H, Konishi S, Tabuchi T (2010) Evaluations of interferon-γ/interleukin-4 ratio and neutrophil/lymphocyte ratio as prognostic indicators in gastric cancer patients. J Surg Oncol 102:742–747. https://doi.org/10.1002/jso.21725
Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ (2005) Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 91:181–184. https://doi.org/10.1002/jso.20329
Wei XL, Wang FH, Zhang DS, Qiu MZ, Ren C, Jin Y, Zhou YX, Wang DS, He MM, Bai L, Wang F, Luo HY, Li YH, Xu RH (2015) A novel inflammation-based prognostic score in esophageal squamous cell carcinoma: the C-reactive protein/albumin ratio. BMC Cancer 15:350. https://doi.org/10.1186/s12885-015-1379-6
Yarchoan M, Albacker LA, Hopkins AC, Montesion M, Murugesan K, Vithayathil TT, Zaidi N, Azad NS, Laheru DA, Frampton GM, Jaffee EM (2019) PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight 4:e126908. https://doi.org/10.1172/jci.insight.126908
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
NS: conception and design, treatment of patients, analysis and interpretation of the data, writing, review and/or revision of the manuscript; TS: conception and design, treatment of patients, analysis and interpretation of the data, writing, review and/or revision of the manuscript; YY: treatment of patients, writing, review and/or revision of the manuscript. HN: writing, review and/or revision of the manuscript; YM: writing, review and/or revision of the manuscript; HC: writing, review and/or revision of the manuscript. All authors participated in manuscript preparation and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Code availability
Not applicable.
Ethics approval
This study was conducted with the approval of the Institutional Review Board (IRB) of Hakodate Goryokaku Hospital (No. 2021–015), and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent to participate
As this was a retrospective study, the IRB waived the requirement for informed consent, but patient confidentiality was protected.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shijubou, N., Sumi, T., Yamada, Y. et al. Immunological and nutritional predictive factors in patients receiving pembrolizumab for the first-line treatment of non-small cell lung cancer. J Cancer Res Clin Oncol 148, 1893–1901 (2022). https://doi.org/10.1007/s00432-022-03941-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-03941-2