Abstract
Background
Smoldering multiple myeloma (SMM) is an intermediate pre-malignant condition with individuals having a distinct risk of progression to overt myeloma. The optimal management option has remained controversial due to the heterogeneous nature of the condition in which progression to overt diseases is variable. The question of who, when, and what to use for the treatment of SMM remains equivocal. We performed a systematic review of randomized controlled trials and summarized the current evidence supporting the best approach to the management of SMM.
Methods
A comprehensive literature search of Medline/PubMed, PubMed Central, Embase, Scopus, Web of Science, Wiley Cochrane Library, CINAHL, clinicaltrial.gov, and conference proceedings of ASCO, ASH, EHA, and ESMO was performed on October 25, 2020. Synthesis of the result was done using narrative analysis.
Result
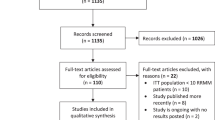
Of the total 1560 identified records, 10 eligible studies involving 1157 patients made up of 580 in the intervention group and 577 in the control group were included in this review. Three early trials of melphalan and prednisone fail to demonstrate any significant impact on disease progression with major toxicities reported. Three trials on bisphosphonate monotherapy show reduced skeletal-related events without any clinical effect on disease progression. Lenalidomide monotherapy or as part of a combination therapy demonstrates superiority in delaying disease progression over observation. Only Lenalidomide and dexamethasone combination demonstrated superior overall survival over observation across the trials.
Conclusion
Trials of lenalidomide in a less intensive approach has shown promise in delaying disease progression and should be investigated further in clinical trials.

Similar content being viewed by others
References
Avilés A, Nambo MJ, Neri N, Castañeda C, Cleto S, Huerta-Guzmán J (2007) Antitumor effect of zoledronic acid in previously untreated patients with multiple myeloma. Med Oncol 24(2):227–230. https://doi.org/10.1007/BF02698044
Avilés A, Neri N, Huerta-Guzmán J, Nambo MJ (2013) Randomized clinical trial of zoledronic acid in multiple myeloma patients undergoing high-dose chemotherapy and stem-cell transplantation. Curr Oncol 20(1):e13-20. https://doi.org/10.3747/co.20.1055
Aviles A, Nambo MJ, Neri N, Castaneda C, Cleto S, Huerta-Guzman J (2007) Antitumor effect of zoledronic acid in previously untreated patients with multiple myeloma. Med Oncol 24:227–230
Brighton TA, Khot A, Harrison SJ, Ghez D, Weiss BM, Kirsch A, Magen H, Gironella M, Oriol A, Streetly M, Kranenburg B, Qin X, Bandekar R, Hu P, Guilfoyle M, Qi M, Nemat S, Goldschmidt H (2019) Randomized, double-blind, placebo-controlled, multicenter study of siltuximab in high-risk smoldering multiple myeloma. Clin Cancer Res 25(13):3772–3775. https://doi.org/10.1158/1078-0432.CCR-18-3470
Bustoros M, Liu CJ, Reyes K et al (2018) Phase II trial of the combination of ixazomib, lenalidomide, and dexamethasone in high-risk smoldering multiple myeloma. Blood 132(suppl 1):804
Conley RB, Adib G, Adler RA, Åkesson KE, Alexander IM, Amenta KC, Blank RD, Brox WT, Carmody EE, Chapman-Novakofski K, Clarke BL, Cody KM, Cooper C, Crandall CJ, Dirschl DR, Eagen TJ, Elderkin AL, Fujita M, Greenspan SL, Halbout P, Hochberg MC, Javaid M, Jeray KJ, Kearns AE, King T, Koinis TF, Koontz JS, Kužma M, Lindsey C, Lorentzon M, Lyritis GP, Michaud LB, Miciano A, Morin SN, Mujahid N, Napoli N, Olenginski TP, Puzas JE, Rizou S, Rosen CJ, Saag K, Thompson E, Tosi LL, Tracer H, Khosla S, Kiel DP (2020) Secondary fracture prevention: consensus clinical recommendations from a multistakeholder coalition. J Bone Miner Res 35(1):36–52. https://doi.org/10.1002/jbmr.3877
D'Arena G, Gobbi PG, Broglia C, Sacchi S, Quarta G, Baldini L, Iannitto E, Falcone A, Guariglia R, Pietrantuono G, Villani O, Martorelli MC, Mansueto G, Sanpaolo G, Cascavilla N, Musto P; Gimema (Gruppo Italiano Malattie Ematologiche Dell'Adulto); Multiple Myeloma Working Party; Gisl (Gruppo Italiano Studio Linfomi) Cooperative Group (2011) Pamidronate versus observation in asymptomatic myeloma: final results with long-term follow-up of a randomized study. Leuk Lymphoma. 52(5):771–775 https://doi.org/10.3109/10428194.2011.553000
Delforge M, Minuk L, Eisenmann JC, Arnulf B, Canepa L, Fragasso A, Leyvraz S, Langer C, Ezaydi Y, Vogl DT, Giraldo-Castellano P, Yoon SS, Zarnitsky C, Escoffre-Barbe M, Lemieux B, Song K, Bahlis NJ, Guo S, Monzini MS, Ervin-Haynes A, Houck V, Facon T (2015) Health-related quality-of-life in patients with newly diagnosed multiple myeloma in the FIRST trial: lenalidomide plus low-dose dexamethasone versus melphalan, prednisone, thalidomide. Haematologica 100(6):826–833. https://doi.org/10.3324/haematol.2014.120121
Dispenzieri A, Kyle RA, Katzmann JA, Therneau TM, Larson D, Benson J, Clark RJ, Melton LJ 3rd, Gertz MA, Kumar SK, Fonseca R, Jelinek DF, Rajkumar SV (2008) Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood 111(2):785–789. https://doi.org/10.1182/blood-2007-08-108357
Durie BG, Salmon SE (1975) A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer 36(3):842–54. https://doi.org/10.1002/1097-0142(197509)36:3<842::aid-cncr2820360303>3.0.co;2-u
Falco P, Bringhen S, Avonto I, Gay F, Morabito F, Boccadoro M, Palumbo A (2007) Melphalan and its role in the management of patients with multiple myeloma. Expert Rev Anticancer Ther 7(7):945–957. https://doi.org/10.1586/14737140.7.7.945
García-Sanz R, Oriol A, Moreno MJ, de la Rubia J, Payer AR, Hernández MT, Palomera L, Teruel AI, Blanchard MJ, Gironella M, Ribas P, Bargay J, Abellá E, Granell M, Ocio EM, Ribera JM, San Miguel JF, Mateos MV; Spanish Myeloma Group (GEM/PETHEMA) (2015) Zoledronic acid as compared with observation in multiple myeloma patients at biochemical relapse: results of the randomized AZABACHE Spanish trial. Haematologica. 100(9):1207–13. https://doi.org/10.3324/haematol.2015.128439
Ghobrial IM, Badros AZ, Vredenburgh JJ et al (2016) Phase II trial of combination of elotuzumab, lenalidomide, and dexamethasone in high-risk smoldering multiple myeloma. Blood 128:976
He Y, Wheatley K, Clark O, Glasmacher A, Ross H, Djulbegovic B (2003) Early versus deferred treatment for early stage multiple myeloma. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD004023
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook
Hjorth M, Hellquist L, Holmberg E, Magnusson B, Rodjer S, Westin J (1993) Initial versus deferred melphalan-prednisone therapy for asymptomatic multiple myeloma stage I - A randomized study. Eur J Haematol 50(2):95–102
Hofmeister C, Chari A, Cohen Y et al (2017) Daratumumab monotherapy for patients with intermediate or high-risk smoldering multiple myeloma (SMM): Centaurus, a randomized, open-label, multicenter phase 2 study. Blood 130(suppl 1):510. https://doi.org/10.1182/blood.V130.Suppl_1.510.510
https://www.cancer.org/cancer/multiple-myeloma/about/key-statistics.html. Accessed on October 29, 2021
International Myeloma Working Group (2003) Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the international myeloma working group. Br J Haematol 121(5):749–757
Joseph NS, Dhodapkar MV, Lonial S (2020) The role of early intervention in high-risk smoldering myeloma. Am Soc Clin Oncol Educ Book 40:1–9. https://doi.org/10.1200/EDBK_278915
Kang HY, Choi EY (2019) Factors influencing quality of life in patients with multiple myeloma. Contemp Nurse 55(2–3):109–121. https://doi.org/10.1080/10376178.2019.1623699
Kumar SK, Gertz MA, Dispenzieri A (2019) Validation of mayo clinic staging system for light chain amyloidosis with high-sensitivity troponin. J Clin Oncol 37(2):171–173. https://doi.org/10.1200/JCO.18.01398
Kyle RA, Greipp PR (1980) Smoldering multiple myeloma. N Engl J Med 302(24):1347–1349. https://doi.org/10.1056/NEJM198006123022405
Kyle RA, Remstein ED, Therneau TM, Dispenzieri A, Kurtin PJ, Hodnefield JM, Larson DR, Plevak MF, Jelinek DF, Fonseca R, Melton LJ 3rd, Rajkumar SV (2007) Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med 356(25):2582–2590. https://doi.org/10.1056/NEJMoa070389
Lakshman A, Rajkumar SV, Buadi FK, Binder M, Gertz MA, Lacy MQ, Dispenzieri A, Dingli D, Fonder AL, Hayman SR, Hobbs MA, Gonsalves WI, Hwa YL, Kapoor P, Leung N, Go RS, Lin Y, Kourelis TV, Warsame R, Lust JA, Russell SJ, Zeldenrust SR, Kyle RA, Kumar SK (2018) Risk stratification of smoldering multiple myeloma incorporating revised IMWG diagnostic criteria. Blood Cancer J 8(6):59. https://doi.org/10.1038/s41408-018-0077-4
Latif T, Chauhan N, Khan R, Moran A, Usmani SZ (2012) Thalidomide and its analogues in the treatment of multiple myeloma. Exp Hematol Oncol 1(1):27. https://doi.org/10.1186/2162-3619-1-27
Liu CJ, Ghobrial IM, Bustoros M et al (2018) Phase II trial of combination of elotuzumab, lenalidomide, and dexamethasone in high-risk smoldering multiple myeloma. Blood 132(suppl 1):154
Lonial S, Jacobus S, Fonseca R, Weiss M, Kumar S, Orlowski RZ, Kaufman JL, Yacoub AM, Buadi FK, O’Brien T, Matous JV, Anderson DM, Emmons RV, Mahindra A, Wagner LI, Dhodapkar MV, Rajkumar SV (2020) Randomized trial of lenalidomide versus observation in smoldering multiple myeloma. J Clin Oncol 38(11):1126–1137. https://doi.org/10.1200/JCO.19.01740
Manasanch E, Jagannath S (2019) A multicenter phase II single arm trial of isatuximab in patients with high risk smoldering multiple myeloma (HRSMM). Blood 184(suppl 1):8118. https://doi.org/10.1182/blood-2019-123205
Martín A, García-Sanz R, Hernández J, Bladé J, Suquía B, Fernández-Calvo J, González M, Mateo G, Orfao A, San Miguel JF (2002) Pamidronate induces bone formation in patients with smouldering or indolent myeloma, with no significant anti-tumour effect. Br J Haematol 118(1):239–242. https://doi.org/10.1046/j.1365-2141.2002.03549.x
Mateos MV, Hernández MT, Giraldo P, de la Rubia J, de Arriba F, Corral LL, Rosiñol L, Paiva B, Palomera L, Bargay J, Oriol A, Prosper F, López J, Arguiñano JM, Quintana N, García JL, Bladé J, Lahuerta JJ, Miguel JS (2016) Lenalidomide plus dexamethasone versus observation in patients with high-risk smouldering multiple myeloma (QuiRedex): long-term follow-up of a randomised, controlled, phase 3 trial. Lancet Oncol 17(8):1127–1136. https://doi.org/10.1016/S1470-2045(16)30124-3
Mateos MV, Kumar S, Dimopoulos MA, González-Calle V, Kastritis E, Hajek R, De Larrea CF, Morgan GJ, Merlini G, Goldschmidt H, Geraldes C, Gozzetti A, Kyriakou C, Garderet L, Hansson M, Zamagni E, Fantl D, Leleu X, Kim BS, Esteves G, Ludwig H, Usmani S, Min CK, Qi M, Ukropec J, Weiss BM, Rajkumar SV, Durie BGM, San-Miguel J (2020) international myeloma working group risk stratification model for smoldering multiple myeloma (SMM). Blood Cancer J 10(10):102. https://doi.org/10.1038/s41408-020-00366-3
Mhaskar R, Kumar A, Miladinovic B, Djulbegovic B (2017) Bisphosphonates in multiple myeloma: an updated network meta-analysis. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003188.pub4
Mikkilineni L, Kochenderfer JN (2021) CAR T cell therapies for patients with multiple myeloma. Nat Rev Clin Oncol 18(2):71–84. https://doi.org/10.1038/s41571-020-0427-6
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA (2015) PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4(1):1. https://doi.org/10.1186/2046-4053-4-1
Musto P, Falcone A, Sanpaolo G, Bodenizza C, Cascavilla N, Melillo L, Scalzulli PR, Dell’Olio M, La Sala A, Mantuano S, Nobile M, Carella AM (2003) Pamidronate reduces skeletal events but does not improve progression-free survival in early-stage untreated myeloma: results of a randomized trial. Leuk Lymphoma 44(9):1545–1548. https://doi.org/10.3109/10428190309178778
Musto P, Petrucci MT, Bringhen S, Guglielmelli T, Caravita T, Bongarzoni V, Andriani A, D'Arena G, Balleari E, Pietrantuono G, Boccadoro M, Palumbo A; GIMEMA (Italian Group for Adult Hematologic Diseases)/Multiple Myeloma Working Party and the Italian Myeloma Network. A multicenter, randomized clinical trial comparing zoledronic acid versus observation in patients with asymptomatic myeloma. Cancer. 2008;113(7):1588–95. https://doi.org/10.1002/cncr.23783. Erratum in: Cancer. 2008;113(10):2835.
Nsiala L, Bobin A, Levy A, Gruchet C, Sabirou F, Gardeney H, Cailly L, Manier S, Moya N, Tomowiak C, Guidez S, Leleu X, Decaux O. Smoldering multiple myeloma: biology, clinical manifestations and management. Leuk Lymphoma. 2021 https://doi.org/10.1080/10428194.2021.1992615
Orlowski RZ, Gercheva L, Williams C, Sutherland H, Robak T, Masszi T, Goranova-Marinova V, Dimopoulos MA, Cavenagh JD, Špička I, Maiolino A, Suvorov A, Bladé J, Samoylova O, Puchalski TA, Reddy M, Bandekar R, van de Velde H, Xie H, Rossi JF (2015) A phase 2, randomized, double-blind, placebo-controlled study of siltuximab (anti-IL-6 mAb) and bortezomib versus bortezomib alone in patients with relapsed or refractory multiple myeloma. Am J Hematol 90(1):42–49. https://doi.org/10.1002/ajh.23868
Pérez-Persona E, Vidriales MB, Mateo G, García-Sanz R, Mateos MV, de Coca AG, Galende J, Martín-Nuñez G, Alonso JM, de Las HN, Hernández JM, Martín A, López-Berges C, Orfao A, San Miguel JF (2007) New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood 110(7):2586–2592. https://doi.org/10.1182/blood-2007-05-088443
Pozzi S, Raje N (2011) The role of bisphosphonates in multiple myeloma: mechanisms, side effects, and the future. Oncologist 16(5):651–662. https://doi.org/10.1634/theoncologist.2010-0225
Rajkumar SV (2020) Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am J Hematol 95(5):548–567. https://doi.org/10.1002/ajh.25791.Erratum.In:AmJHematol.2020;95(11):1444
Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E, Richardson P, Landgren O, Paiva B, Dispenzieri A, Weiss B, LeLeu X, Zweegman S, Lonial S, Rosinol L, Zamagni E, Jagannath S, Sezer O, Kristinsson SY, Caers J, Usmani SZ, Lahuerta JJ, Johnsen HE, Beksac M, Cavo M, Goldschmidt H, Terpos E, Kyle RA, Anderson KC, Durie BG, Miguel JF (2014) International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 15(12):e538–e548. https://doi.org/10.1016/S1470-2045(14)70442-5
Rajkumar SV, Landgren O, Mateos MV (2015) Smoldering multiple myeloma. Blood 125(20):3069–3075. https://doi.org/10.1182/blood-2014-09-568899
Riccardi A, Ucci G, Luoni R et al (1994) Treatment of multiple myeloma according to the extension of the disease: a prospective, randomized study comparing a less with a more aggressive cystostatic policy. Cooperative group of study and treatment of multiple myeloma. Br J Cancer 70(6):1203–1210. https://doi.org/10.1038/bjc.1994.474
Riccardi A, Mora O, Tinelli C et al (2000) Long-term survival of stage I multiple myeloma given chemotherapy just after diagnosis or at progression of the disease: a multicentre randomized study. Cooperative group of study and treatment of multiple myeloma. Br J Cancer 82(7):1254–1260. https://doi.org/10.1054/bjoc.1999.1087
San Miguel J, Mateos MV, Gonzalez V et al (2019) Updated risk stratification model for smoldering multiple myeloma (SMM) incorporating the revised IMWG diagnostic criteria. J Clin Oncol 37:15s. https://doi.org/10.1200/JCO.2019.37.15_suppl.8000
San-Miguel J, Bladé J, Shpilberg O, Grosicki S, Maloisel F, Min CK, Polo Zarzuela M, Robak T, Prasad SV, Tee Goh Y, Laubach J, Spencer A, Mateos MV, Palumbo A, Puchalski T, Reddy M, Uhlar C, Qin X, van de Velde H, Xie H, Orlowski RZ (2014) Phase 2 randomized study of bortezomib-melphalan-prednisone with or without siltuximab (anti-IL-6) in multiple myeloma. Blood 123(26):4136–4142. https://doi.org/10.1182/blood-2013-12-546374.Erratum.In:Blood.2014;124(7):1201
Stewart AK, Jacobus S, Fonseca R, Weiss M, Callander NS, Chanan-Khan AA, Rajkumar SV (2015) Melphalan, prednisone, and thalidomide vs melphalan, prednisone, and lenalidomide (ECOG E1A06) in untreated multiple myeloma. Blood 126(11):1294–1301. https://doi.org/10.1182/blood-2014-12-613927
Walker BA, Wardell CP, Melchor L, Brioli A, Johnson DC, Kaiser MF, Mirabella F, Lopez-Corral L, Humphray S, Murray L, Ross M, Bentley D, Gutiérrez NC, Garcia-Sanz R, San Miguel J, Davies FE, Gonzalez D, Morgan GJ (2014) Intraclonal heterogeneity is a critical early event in the development of myeloma and precedes the development of clinical symptoms. Leukemia 28(2):384–390. https://doi.org/10.1038/leu.2013.199
Witzig TE, Laumann KM, Lacy MQ, Hayman SR, Dispenzieri A, Kumar S, Reeder CB, Roy V, Lust JA, Gertz MA, Greipp PR, Hassoun H, Mandrekar SJ, Rajkumar SV (2013) A phase III randomized trial of thalidomide plus zoledronic acid versus zoledronic acid alone in patients with asymptomatic multiple myeloma. Leukemia 27(1):220–225. https://doi.org/10.1038/leu.2012.236
Zhan F, Hardin J, Kordsmeier B, Bumm K, Zheng M, Tian E, Sanderson R, Yang Y, Wilson C, Zangari M, Anaissie E, Morris C, Muwalla F, van Rhee F, Fassas A, Crowley J, Tricot G, Barlogie B, Shaughnessy J Jr (2002) Global gene expression profiling of multiple myeloma, monoclonal gammopathy of undetermined significance, and normal bone marrow plasma cells. Blood 99(5):1745–1757. https://doi.org/10.1182/blood.v99.5.1745
Zhao S, Choi M, Heuck C, Mane S, Barlogie B, Lifton RP, Dhodapkar MV (2014) Serial exome analysis of disease progression in premalignant gammopathies. Leukemia 28(7):1548–1552. https://doi.org/10.1038/leu.2014.59
Zhao AL, Shen KN, Wang JN, Huo LQ, Li J, Cao XX (2019) Early or deferred treatment of smoldering multiple myeloma: a meta-analysis on randomized controlled studies. Cancer Manag Res 11:5599–5611. https://doi.org/10.2147/CMAR.S205623
Funding
This research did not receive any grant from funding agencies in the public, not-for-profit or commercial sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix I
Search Strategy.
Asymptomatic multiple myeloma or smoldering myeloma or smoldering multiple myeloma.
AND
Clinical trial or randomized controlled trial or intervention or therapy or drug treatment or Lenalidomide or Dexamethasone or Daratumumab or Thalidomide or Zoledronic acid or Carfilzomib or Siltuximab.
AND
Controlled or observation or no treatment or no intervention or Placebo.
AND
Disease progression or active multiple myeloma.
Search example:
"smoldering multiple myeloma"[MeSH Terms] OR ("smoldering"[All Fields] AND "multiple"[All Fields] AND "myeloma"[All Fields]) OR "smoldering multiple myeloma"[All Fields] OR ("smoldering"[All Fields] AND "myeloma"[All Fields]) OR "smoldering myeloma"[All Fields].
Appendix II

Rights and permissions
About this article
Cite this article
Ojo, A.S., Ojukwu, S.G., Asemota, J. et al. The effect of intervention versus watchful waiting on disease progression and overall survival in smoldering multiple myeloma: a systematic review of randomized controlled trials. J Cancer Res Clin Oncol 148, 897–911 (2022). https://doi.org/10.1007/s00432-022-03920-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-03920-7