Abstract
Purpose
We aimed to investigate whether induction chemotherapy with less than four courses is as effective as induction chemotherapy with more than four courses in non-small cell lung cancer (NSCLC) patients receiving chemoimmunotherapy.
Methods
We retrospectively enrolled 249 patients with NSCLC who received chemoimmunotherapy at 12 centers in Japan between January and December 2019. The patient group that completed less than four courses owing to adverse events (AEs), and received subsequent maintenance therapy was compared to the group that received at least four courses of induction chemotherapy followed by maintenance therapy.
Results
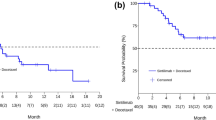
On univariate and multivariate analyses, the patient group that transitioned to maintenance therapy after completing less than four courses of induction chemotherapy had significantly shorter progression-free survival (PFS) than those who completed at least four courses (hazard ratio [HR] 2.15, 95% confidence interval: 1.38–3.37, p < 0.001 and HR 2.32, 95% confidence interval: 1.40–3.84, p = 0.001, respectively). There was no obvious difference in PFS between the group in which induction chemotherapy ended in two or three courses leading to partial or complete response, and the group that continued at least four courses of induction chemotherapy (log-rank test p = 0.53).
Conclusion
Treatment efficacy may be maintained if induction chemotherapy is completed in less than four courses owing to development of AEs, and is administered for more than two courses with partial or complete response; efficacy is maintained even on transitioning to maintenance therapy.



Similar content being viewed by others
Availability of data and materials
The datasets generated during the current study are not publicly available due to ethical considerations, but are available from the corresponding author on reasonable request.
References
Borghaei H, Paz-Ares L, Horn L et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373(17):1627–1639. https://doi.org/10.1056/NEJMoa1507643
Bracci L, Schiavoni G, Sistigu A et al (2014) Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ 21:15–25. https://doi.org/10.1038/cdd.2013.67
Cortellini A, Chiari R, Ricciuti B et al (2019) Correlations between the immune-related adverse events spectrum and efficacy of anti-PD1 immunotherapy in NSCLC patients. Clin Lung Cancer 20:237–247. https://doi.org/10.1016/j.cllc.2019.02.006
Gandhi L, Rodríguez-Abreu D, Gadgeel S et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378:2078–2092. https://doi.org/10.1056/NEJMoa1801005
Grangeon M, Tomasini P, Chaleat S et al (2019) Association between immune-related adverse events and efficacy of immune checkpoint inhibitors in non-small-cell lung cancer. Clin Lung Cancer 20:201–207. https://doi.org/10.1016/j.cllc.2018.10.002
Haratani K, Hayashi H, Chiba Y et al (2018) Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 4:374–378. https://doi.org/10.1001/jamaoncol.2017.2925
Hellmann MD, Paz-Ares L, Bernabe Caro R et al (2019) Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med 381:2020–2031. https://doi.org/10.1056/NEJMoa1910231
Inoue H, Tsutsumi H, Tanaka K et al (2021) Increased plasma levels of damage-associated molecular patterns during systemic anticancer therapy in patients with advanced lung cancer. Transl Lung Cancer Res 10(6):2475–2486. https://doi.org/10.21037/tlcr-21-92
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl 48:452–458. https://doi.org/10.1038/bmt.2012.244
Katayama Y, Shimamoto T, Yamada T et al (2019) Retrospective efficacy analysis of immune checkpoint inhibitor rechallenge in patients with non-small cell lung cancer. J Clin Med 9:102. https://doi.org/10.3390/jcm9010102
Krysko DV, Garg AD, Kaczmarek A et al (2012) Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer 12(12):860–875. https://doi.org/10.1038/nrc3380
Paz-Ares L, Luft A, Vicente D et al (2018) Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379:2040–2051. https://doi.org/10.1056/NEJMoa1810865
Reck M, Ciuleanu TM, Dols MC et al (2020) Nivolumab (NIVO) + ipilimumab (IPI) + 2 cycles of platinum-doublet chemotherapy (chemo) vs 4 cycles chemo as first-line (1L) treatment (tx) for stage IV/recurrent non-small cell lung cancer (NSCLC): CheckMate 9LA. J Clin Oncol 38:9501. https://doi.org/10.1200/JCO.2020.38.15_suppl.9501
Sato K, Akamatsu H, Murakami E et al (2018) Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer 115:71–74. https://doi.org/10.1016/j.lungcan.2017.11.019
Socinski MA, Jotte RM, Cappuzzo F et al (2018) Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378:2288–2301. https://doi.org/10.1056/NEJMoa1716948
Spigel DR, McCleod M, Jotte RM et al (2019) Safety, efficacy, and patient-reported health-related quality of life and symptom burden with nivolumab in patients with advanced non-small cell lung cancer, including patients aged 70 years or older or with poor performance status (CheckMate 153). J Thorac Oncol 149:1628. https://doi.org/10.1016/j.jtho.2019.05.010
West H, McCleod M, Hussein M et al (2019) Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20:924–937. https://doi.org/10.1016/S1470-2045(19)30167-6
Acknowledgements
We would like to sincerely thank the patients, their families, and all investigators involved in this study. Additionally, we would like to thank Editage (www.editage.jp) for assistance with English-language editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
KM, JU, TT, and KT contributed to the study conception and design. KM, CT, TT, Takahiro Y, OH, YC, Takashi Y, AN, HY, MT, YG, MH, and TK obtained the clinical data. Data were interpreted by KM, JU, YM, MI, YK, Tadaaki Y, and KT. The manuscript was prepared by KM and JU. The final version of the manuscript was read and approved by all the authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Code availability
Not applicable.
Ethical approval
The study protocol was approved by the ethics committees of each hospital, including the Kyoto Prefectural University of Medicine (Approval No. ERB-C-1803).
Consent to participate
As this was a retrospective study, the need for informed consent was waived and the official website was used for opt-out; this was approved by the Ethics Committees of each hospital.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Morimoto, K., Uchino, J., Yokoi, T. et al. Early discontinuation of induction therapy in chemoimmunotherapy as an effective alternative to the standard regimen in patients with non-small cell lung cancer: a retrospective study. J Cancer Res Clin Oncol 148, 2437–2446 (2022). https://doi.org/10.1007/s00432-021-03782-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03782-5