Abstract
Purpose
Studies show that axillary surgery can be potentially omitted in certain breast cancer patients who achieve breast pathologic complete response (pCR) after neoadjuvant systemic therapy (NST). However, potential differences between the ypT0 and ypTis subgroups remain to be explored. Furthermore, whether axillary surgery can be omitted in patients with clinically assessed positive axillary lymph nodes (cN+) remains unknown. This study was to evaluate the status of axillary lymph nodes for patients who achieved breast pCR after NST in the real-world study.
Methods
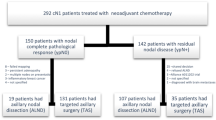
This retrospective cohort study included 258 patients with early or locally advanced breast cancer who underwent breast and axillary surgery after NST. Clinical and pathologic data were compared between patients with breast pCR (ypT0/is) and those without breast pCR.
Results
The rate of breast pCR after NST was 27.1% (70/258). Among the patients with initial cN0, the rate of axillary pCR was similar between the breast pCR and breast non-pCR groups (100% vs. 85.7%, P = 0.1543). Among those with breast pCR, the rate of axillary pCR was 100% in both the ypT0 and ypTis subgroups. Furthermore, among those with initial cN+, the rate of axillary pCR was higher in the breast pCR group than in the breast non-pCR group (82.7% vs. 22.9%, P < 0.0001). Among the patients with breast pCR, the rate of axillary pCR was higher in the ypT0 subgroup than in the ypTis subgroup (94.3% vs. 58.8%, P = 0.0034).
Conclusion
Axillary surgery may potentially be omitted in patients with initial cN0 who achieve breast pCR (ypT0/is), and may also be considered for omission in patients with initial cN+ who achieve ypT0 (not ypTis).
Similar content being viewed by others
Availability of data and material
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Amiei S, van Nijnatten TJA, de Munck L, Keymeulen KBMI, Simons JM, Kooreman LFS et al (2020) Correlation between pathologic complete response in the breast and absence of axillary lymph node metastases after neoadjuvant systemic therapy. Ann Surg 271:574–580
Barron AU, Hoskin TL, Day CN, Hwang ES, Kuerer HM, Boughey JC (2018) Association of low nodal positivity rate among patients with ERBB2-positive or triple-negative breast cancer and breast pathologic complete response to neoadjuvant chemotherapy. JAMA Surg 153:1120–1126
Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B et al (2013) Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 310:1455–1461
Choi HJ, Ryu JM, Kim I, Gil-Gil M, Perez-Montero H, Fernandez-Montolí E et al (2019) Prediction of axillary pathologic response with breast pathologic complete response after neoadjuvant chemotherapy. Breast Cancer Res Treat 176:591–596
Gluz O, Nitz U, Liedtke C, Christgen M, Grischke EM, Forstbauer H et al (2018) Comparison of neoadjuvant nab-paclitaxel+carboplatin vs nab-paclitaxel+gemcitabine in triple-negative breast cancer: randomized WSG-ADAPT-TN trial results. J Natl Cancer Inst 110:628–637
Heil J, Kummel S, Schaefgen B, Paepke S, Thomssen C, Rauch G et al (2015) Diagnosis of pathological complete response to neoadjuvant chemotherapy in breast cancer by minimal invasive biopsy techniques. Br J Cancer 113:1565–1570
Hennessy BT, Hortobagyi GN, Rouzier R, Kuerer H, Sneige N, Buzdar AU et al (2005) Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol 23:9304–9311
Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E (2012) Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer 48:3342–3354
Kolberg HC, Kühn T, Krajewska M, Bauerfeind I, Fehm TN, Fleige B et al (2020) Residual axillary burden after neoadjuvant chemotherapy (NACT) in early breast cancer in patients with a priori clinically occult nodal metastases—a transSENTINA analysis. Geburtshilfe Frauenheilkd 80(12):1229–1236
Kong X, Moran MS, Zhang N, Haffty B, Yang Q (2011) Meta-analysis confirms achieving pathological complete response after neoadjuvant chemotherapy predicts favourable prognosis for breast cancer patients. Eur J Cancer 47:2084–2090
Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP et al (2010) Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 11:927–933
Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G et al (2013) Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 14:609–618
Kuerer HM, Rauch GM, Krishnamurthy S, Adrada BE, Caudle AS, DeSnyder SM et al (2018) A clinical feasibility trial for identification of exceptional responders in whom breast cancer surgery can be eliminated following neoadjuvant systemic therapy. Ann Surg 267:946–951
Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM et al (2006) Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC trial. J Natl Cancer Inst 98:599–609
Mougalian SS, Hernandez M, Lei X, Lynch S, Kuerer HM, Symmans WF et al (2016) Ten-year outcomes of patients with breast cancer with cytologically confirmed axillary lymph node metastases and pathologic complete response after primary systemic chemotherapy. JAMA Oncol 2:508–516
Shen J, Gilcrease MZ, Babiera GV, Ross MI, Meric-Bernstam F, Feig BW et al (2007) Feasibility and accuracy of sentinel lymph node biopsy after preoperative chemotherapy in breast cancer patients with documented axillary metastases. Cancer 109:1255–1263
Tadros AB, Yang WT, Krishnamurthy S, Rauch GM, Smith BD, Valero V et al (2017) Identification of patients with documented pathologic complete response in the breast after neoadjuvant chemotherapy for omission of axillary surgery. JAMA Surg 152:665–670
von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA et al (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30:1796–1804
Acknowledgements
This research was supported in part by Jiangsu Province Six Talents Summit Project (WSW-001), Youth Talent Project (FRC201308), Jiangsu Women and Children Health Research Project (F201761), Chinese Society of Clinical Oncology Foundation (Y-sy2018-077, Y-JS2019-096), National Natural Science Foundation of China (81302305), Natural Science Foundation of Jiangsu Province (BK20131027), Jiangsu Province Women and Children Health Scientific Research Project (F201821).
Funding
The authors received no financial support for the research, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
XZ and JW designed this study and conducted quality control on it. RC, YL, and XS participated in the analysis of data and drafted the manuscript. SL, QZ, LX, YX, WZ and XH were responsible for data collection. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
All the procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and those of the Helsinki Declaration. This work was approved by the ethics and research committee of the First Affiliated Hospital of Nanjing Medical University (2020-SR-081), and informed consent was obtained from all the participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, R., Li, S., Li, Y. et al. Can axillary surgery be omitted in patients with breast pathologic complete response after neoadjuvant systemic therapy for breast cancer? A real-world retrospective study in China. J Cancer Res Clin Oncol 147, 3495–3501 (2021). https://doi.org/10.1007/s00432-021-03763-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03763-8