Abstract
Background
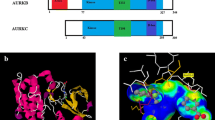
Ensuring genetic integrity is essential during the cell cycle to avoid aneuploidy, one of the underlying causes of malignancies. Aurora kinases are serine/threonine kinase that play a vital role in maintaining the genomic integrity of the cells. There are three forms of aurora kinases in the mammalian cells, which are highly conserved and act together with several other proteins to control chromosome alignment and its equal distribution to daughter cells in mitosis and meiosis.
Methods
We provide here a detailed analysis of Aurora B kinase (ABK) in terms of its expression, structure, function, disease association and potential therapeutic implications.
Results
ABK plays an instrumental in mitotic entry, chromosome condensation, spindle assembly, cytokinesis, and abscission. Small-molecule inhibitors of ABK are designed and synthesized to control cancer progression. A detailed understanding of ABK pathophysiology in different cancers is of great significance in designing and developing effective therapeutic strategies.
Conclusion
In this review, we have discussed the physiological significance of ABK followed by its role in cancer progression. We further highlighted available small-molecule inhibitors to control the tumor proliferation and their mechanistic insights.




Similar content being viewed by others
Abbreviations
- ABK:
-
Aurora B kinase
- CLK:
-
Cdc-like kinase
- shRNAs:
-
Short-hairpin RNAs
- CPC:
-
Chromosomal passenger complex
- CDK:
-
Cyclin-dependent kinase
- SAC:
-
Spindle assembly checkpoint
References
Adams RR, Carmena M, Earnshaw WC (2001) Chromosomal passengers and the (aurora) ABCs of mitosis. Trends Cell Biol 11:49–54
Araki K, Nozaki K, Ueba T, Tatsuka M, Hashimoto N (2004) High expression of Aurora-B/Aurora and Ipl1-like midbody-associated protein (AIM-1) in astrocytomas. J Neurooncol 67:53–64
Azzariti A, Bocci G, Porcelli L, Fioravanti A, Sini P, Simone G, Quatrale A, Chiarappa P, Mangia A, Sebastian S (2011) Aurora B kinase inhibitor AZD1152: determinants of action and ability to enhance chemotherapeutics effectiveness in pancreatic and colon cancer. Br J Cancer 104:769–780
Bebbington D, Binch H, Charrier J-D, Everitt S, Fraysse D, Golec J, Kay D, Knegtel R, Mak C, Mazzei F (2009) The discovery of the potent aurora inhibitor MK-0457 (VX-680). Bioorg Med Chem Lett 19:3586–3592
Bischoff JR, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, Schryver B, Flanagan P, Clairvoyant F, Ginther C (1998) A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J 17:3052–3065
Bogen D, Wei JS, Azorsa DO, Ormanoglu P, Buehler E, Guha R, Keller JM, Griner LAM, Ferrer M, Song YK (2015) Aurora B kinase is a potent and selective target in MYCN-driven neuroblastoma. Oncotarget 6:35247
Borah NA, Reddy MM (2021) Aurora kinase B inhibition: a potential therapeutic strategy for cancer. Molecules 26(7):1981
Carlton JG, Caballe A, Agromayor M, Kloc M, Martin-Serrano J (2012) ESCRT-III governs the Aurora B–mediated abscission checkpoint through CHMP4C. Science 336:220–225
Carmena M, Earnshaw WC (2003) The cellular geography of aurora kinases. Nat Rev Mol Cell Biol 4:842–854
Carmena M, Ruchaud S, Earnshaw WC (2009) Making the Auroras glow: regulation of Aurora A and B kinase function by interacting proteins. Curr Opin Cell Biol 21:796–805
Casorzo L, Dell’Aglio C, Sarotto I, Risio M (2015) Aurora kinase A gene copy number is associated with the malignant transformation of colorectal adenomas but not with the serrated neoplasia progression. Hum Pathol 46:411–418
Cha T-L, Chuang M-J, Wu S-T, Sun G-H, Chang S-Y, Yu D-S, Huang S-M, Huan SK-H, Cheng T-C, Chen T-T (2009) Dual degradation of aurora A and B kinases by the histone deacetylase inhibitor LBH589 induces G2-M arrest and apoptosis of renal cancer cells. Clin Cancer Res 15:840–850
Chan CS, Botstein D (1993) Isolation and characterization of chromosome-gain and increase-in-ploidy mutants in yeast. Genetics 135:677–691
Chan FL, Vinod B, Novy K, Schittenhelm RB, Huang C, Udugama M, Nunez-Iglesias J, Lin JI, Hii L, Chan J (2017) Aurora Kinase B, a novel regulator of TERF1 binding and telomeric integrity. Nucleic Acids Res 45:12340–12353
Chen Y-J, Chen C-M, Twu N-F, Yen M-S, Lai C-R, Wu H-H, Wang P-H, Yuan C-C (2009) Overexpression of Aurora B is associated with poor prognosis in epithelial ovarian cancer patients. Virchows Arch 455:431–440
Chen C, Zhang Z, Cui P, Liao Y, Zhang Y, Yao L, Rui R, Ju S (2017) Phosphorylation of histone H3 on Ser-10 by Aurora B is essential for chromosome condensation in porcine embryos during the first mitotic division. Histochem Cell Biol 148:73–83
Cheng L, Zhang J, Ahmad S, Rozier L, Yu H, Deng H, Mao Y (2011) Aurora B regulates formin mDia3 in achieving metaphase chromosome alignment. Dev Cell 20:342–352
Chieffi P, Troncone G, Caleo A, Libertini S, Linardopoulos S, Tramontano D, Portella G (2004) Aurora B expression in normal testis and seminomas. J Endocrinol 181:263–270
Chieffi P, Cozzolino L, Kisslinger A, Libertini S, Staibano S, Mansueto G, De Rosa G, Villacci A, Vitale M, Linardopoulos S (2006) Aurora B expression directly correlates with prostate cancer malignancy and influence prostate cell proliferation. Prostate 66:326–333
Coumar MS, Cheung CH, Chang JY, Hsieh HP (2009) Advances in Aurora kinase inhibitor patents. Expert Opin Ther Pat 19:321–356
D’Assoro AB, Haddad T, Galanis E (2016) Aurora-A kinase as a promising therapeutic target in cancer. Front Oncol 5:295
Dar AA, Goff LW, Majid S, Berlin J, El-Rifai W (2010) Aurora kinase inhibitors-rising stars in cancer therapeutics? Mol Cancer Ther 9:268–278
D’Assoro AB, Lingle WL, Salisbury JL (2002) Centrosome amplification and the development of cancer. Oncogene 21:6146–6153
Davidson B, Nymoen DA, Elgaaen BV, Staff AC, Tropé CG, Kærn J, Reich R, Falkenthal TEH (2014) BUB1 mRNA is significantly co-expressed with AURKA and AURKB mRNA in advanced-stage ovarian serous carcinoma. Virchows Arch 464:701–707
Dawson MA, Curry JE, Barber K, Beer PA, Graham B, Lyons JF, Richardson CJ, Scott MA, Smyth T, Squires MS (2010) AT9283, a potent inhibitor of the Aurora kinases and Jak2, has therapeutic potential in myeloproliferative disorders. Br J Haematol 150:46–57
de Almeida Magalhães T, de Sousa GR, Cruzeiro GAV, Tone LG, Valera ET, Borges KS (2020) The therapeutic potential of Aurora kinases targeting in glioblastoma: from preclinical research to translational oncology. J Mol Med 98:495–512
Defaux J, Antoine M, Logé C, Le Borgne M, Schuster T, Seipelt I, Aicher B, Teifel M, Günther E, Gerlach M (2014) Discovery of (7-aryl-1, 5-naphthyridin-2-yl) ureas as dual inhibitors of ERK2 and Aurora B kinases with antiproliferative activity against cancer cells. Bioorg Med Chem Lett 24:3748–3752
Diamond JR, Bastos BR, Hansen RJ, Gustafson DL, Eckhardt SG, Kwak EL, Pandya SS, Fletcher GC, Pitts TM, Kulikowski GN (2011) Phase I safety, pharmacokinetic, and pharmacodynamic study of ENMD-2076, a novel angiogenic and Aurora kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res 17:849–860
Eshleman HD, Morgan DO (2014) Sgo1 recruits PP2A to chromosomes to ensure sister chromatid bi-orientation during mitosis. J Cell Sci 127:4974–4983
Eyers PA, Churchill ME, Maller JL (2005) The Aurora A and Aurora B protein kinases: a single amino acid difference controls intrinsic activity and activation by TPX2. Cell Cycle 4:784–789
Fletcher GC, Brokx RD, Denny TA, Hembrough TA, Plum SM, Fogler WE, Sidor CF, Bray MR (2011) ENMD-2076 Is an Orally Active Kinase Inhibitor with Antiangiogenic and Antiproliferative Mechanisms of Action. Mol Cancer Ther 10(1):126–137. https://doi.org/10.1158/1535-7163.MCT-10-0574
Fu J, Bian M, Jiang Q, Zhang C (2007) Roles of Aurora kinases in mitosis and tumorigenesis. Mol Cancer Res 5:1–10
Fu J, Bian M, Liu J, Jiang Q, Zhang C (2009) A single amino acid change converts Aurora-A into Aurora-B-like kinase in terms of partner specificity and cellular function. Proc Natl Acad Sci 106:6939–6944
Galetta D, Cortes-Dericks L (2020) Promising therapy in lung cancer: spotlight on aurora kinases. Cancers 12:3371
García-Fernández E, De Diego JI, Collantes-Bellido E, Mendiola M, Prim MP, Pérez-Fernández E, Miguel-Martín M, Nistal M, Hardisson D (2011) Aurora B kinase expression in laryngeal squamous cell carcinoma and its prognostic implications. Histopathology 58:368–376
Gassmann R, Carvalho A, Henzing AJ, Ruchaud S, Hudson DF, Honda R, Nigg EA, Gerloff DL, Earnshaw WC (2004) Borealin a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J Cell Biol 166:179–191
Geuns-Meyer S, Cee VJ, Deak HL, Du B, Hodous BL, Nguyen HN, Olivieri PR, Schenkel LB, Vaida KR, Andrews P (2015) Discovery of N-(4-(3-(2-aminopyrimidin-4-yl) pyridin-2-yloxy) phenyl)-4-(4-methylthiophen-2-yl) phthalazin-1-amine (AMG 900), a highly selective, orally bioavailable inhibitor of aurora kinases with activity against multidrug-resistant cancer cell lines. J Med Chem 58:5189–5207
Giet R, Glover DM (2001) Drosophila aurora B kinase is required for histone H3 phosphorylation and condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis. J Cell Biol 152:669–682
Girdler F, Gascoigne KE, Eyers PA, Hartmuth S, Crafter C, Foote KM, Keen NJ, Taylor SS (2006) Validating Aurora B as an anti-cancer drug target. J Cell Sci 119:3664–3675
Glover DM, Leibowitz MH, McLean DA, Parry H (1995) Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell 81:95–105
Gold MG, Barford D, Komander D (2006) Lining the pockets of kinases and phosphatases. Curr Opin Struct Biol 16:693–701
Goldenson B, Crispino JD (2015) The aurora kinases in cell cycle and leukemia. Oncogene 34:537–545
Goto H, Yasui Y, Nigg EA, Inagaki M (2002) Aurora-B phosphorylates Histone H3 at serine28 with regard to the mitotic chromosome condensation. Genes Cells 7:11–17
Graux C, Sonet A, Maertens J, Duyster J, Greiner J, Chalandon Y, Martinelli G, Hess D, Heim D, Giles FJ (2013) A phase I dose-escalation study of MSC1992371A, an oral inhibitor of aurora and other kinases, in advanced hematologic malignancies. Leuk Res 37:1100–1106
Gu S, Zaidi S, Hassan MI, Mohammad T, Malta TM, Noushmehr H, Nguyen B, Crandall KA, Srivastav J, Obias V et al (2020) Mutated CEACAMs disrupt transforming growth factor beta signaling and alter the intestinal microbiome to promote colorectal carcinogenesis. Gastroenterology 158:238–252
Gully CP, Velazquez-Torres G, Shin J-H, Fuentes-Mattei E, Wang E, Carlock C, Chen J, Rothenberg D, Adams HP, Choi HH (2012) Aurora B kinase phosphorylates and instigates degradation of p53. Proc Natl Acad Sci 109:E1513–E1522
Gupta P, Taiyab A, Hussain A, Alajmi MF, Islam A, Hassan MI (2021) Targeting the Sphingosine Kinase/Sphingosine-1-Phosphate signaling axis in drug discovery for cancer therapy. Cancers 13(8):1898
Gurley LR, Danna JA, Barham SS, Deaven LL, Tobey RA (1978) Histone phosphorylation and chromatin structure during mitosis in Chinese hamster cells. Eur J Biochem 84:1–15
Hans F, Skoufias DA, Dimitrov S, Margolis RL (2009) Molecular distinctions between Aurora A and B: a single residue change transforms Aurora A into correctly localized and functional Aurora B. Mol Biol Cell 20:3491–3502
Hay AE, Murugesan A, DiPasquale AM, Kouroukis T, Sandhu I, Kukreti V, Bahlis NJ, Lategan J, Reece DE, Lyons JF (2016) A phase II study of AT9283, an aurora kinase inhibitor, in patients with relapsed or refractory multiple myeloma: NCIC clinical trials group IND. 191. Leuk Lymphoma 57:1463–1466
Helfrich BA, Kim J, Gao D, Chan DC, Zhang Z, Tan A-C, Bunn PA (2016) Barasertib (AZD1152), a small molecule Aurora B inhibitor, inhibits the growth of SCLC cell lines in vitro and in vivo. Mol Cancer Ther 15:2314–2322
Hindriksen S, Lens S, Hadders MA (2017) The ins and outs of Aurora B inner centromere localization. Front Cell Dev Biol 5:112
Hirano T (2006) At the heart of the chromosome: SMC proteins in action. Nat Rev Mol Cell Biol 7:311–322
Honma K, Nakanishi R, Nakanoko T, Ando K, Saeki H, Oki E, Iimori M, Kitao H, Kakeji Y, Maehara Y (2014) Contribution of Aurora-A and-B expression to DNA aneuploidy in gastric cancers. Surg Today 44:454–461
Hutterer A, Berdnik D, Wirtz-Peitz F, Žigman M, Schleiffer A, Knoblich JA (2006) Mitotic activation of the kinase Aurora-A requires its binding partner Bora. Dev Cell 11:147–157
Jeong Y, Lee J, Ryu J-S (2016) Design, synthesis, and evaluation of hinge-binder tethered 1, 2, 3-triazolylsalicylamide derivatives as Aurora kinase inhibitors. Bioorg Med Chem 24:2114–2124
Jiang J, Wang J, Yue M, Cai X, Wang T, Wu C, Su H, Wang Y, Han M, Zhang Y (2020) Direct phosphorylation and stabilization of MYC by Aurora B kinase promote T-cell leukemogenesis. Cancer Cell 37:200-215.e205
Joukov V, De Nicolo A (2018) Aurora-PLK1 cascades as key signaling modules in the regulation of mitosis. Sci Signaling 11:eaar4194
Kanda A, Kawai H, Suto S, Kitajima S, Sato S, Takata T, Tatsuka M (2005) Aurora-B/AIM-1 kinase activity is involved in Ras-mediated cell transformation. Oncogene 24:7266–7272
Kantarjian HM, Schuster MW, Jain N, Advani A, Jabbour E, Gamelin E, Rasmussen E, Juan G, Anderson A, Chow VF (2017) A phase 1 study of AMG 900, an orally administered pan-aurora kinase inhibitor, in adult patients with acute myeloid leukemia. Am J Hematol 92:660–667
Karthigeyan D, Prasad SBB, Shandilya J, Agrawal S, Kundu TK (2011) Biology of Aurora A kinase: implications in cancer manifestation and therapy. Med Res Rev 31:757–793
Kasam RK, Ghandikota S, Soundararajan D, Reddy GB, Huang SK, Jegga AG, Madala SK (2020) Inhibition of Aurora Kinase B attenuates fibroblast activation and pulmonary fibrosis. EMBO Mole Med 12:12131
Katayama H, Brinkley WR, Sen S (2003) The Aurora kinases: role in cell transformation and tumorigenesis. Cancer Metastasis Rev 22:451–464
Khan NS, Khan P, Ansari MF, Srivastava S, Hasan GM, Husain M, Hassan MI (2018) Thienopyrimidine-chalcone hybrid molecules inhibit fas-activated serine/threonine Kinase: an approach to ameliorate antiproliferation in human breast cancer cells. Mol Pharm 15:4173–4189
Kimura M, Uchida C, Takano Y, Kitagawa M, Okano Y (2004) Cell cycle-dependent regulation of the human aurora B promoter. Biochem Biophys Res Commun 316:930–936
Knauf JA, Ouyang B, Knudsen ES, Fukasawa K, Babcock G, Fagin JA (2006) Oncogenic RAS induces accelerated transition through G2/M and promotes defects in the G2 DNA damage and mitotic spindle checkpoints. J Biol Chem 281:3800–3809
Komaki S, Takeuchi H, Hamamura Y, Heese M, Hashimoto T, Schnittger A (2020) Functional analysis of the plant chromosomal passenger complex. Plant Physiol 183:1586–1599
Li M, Liu H, Zhao Q, Han S, Zhou L, Liu W, Li W, Gao F (2021) Targeting Aurora B kinase with Tanshinone IIA suppresses tumor growth and overcomes radioresistance. Cell Death Dis 12:1–14
Lin Z-Z, Jeng Y-M, Hu F-C, Pan H-W, Tsao H-W, Lai P-L, Lee P-H, Cheng A-L, Hsu H-C (2010) Significance of Aurora B overexpression in hepatocellular carcinoma. Aurora B Overexpression in HCC. BMC Cancer 10:1–14
Lin X, Xiang X, Hao L, Wang T, Lai Y, Abudoureyimu M, Zhou H, Feng B, Chu X, Wang R (2020) The role of Aurora-A in human cancers and future therapeutics. Am J Cancer Res 10:2705
Lindon C, Grant R, Min M (2016) Ubiquitin-mediated degradation of aurora kinases. Front Oncol 5:307
Lipp JJ, Hirota T, Poser I, Peters J-M (2007) Aurora B controls the association of condensin I but not condensin II with mitotic chromosomes. J Cell Sci 120:1245–1255
Littlepage LE, Wu H, Andresson T, Deanehan JK, Amundadottir LT, Ruderman JV (2002) Identification of phosphorylated residues that affect the activity of the mitotic kinase Aurora-A. Proc Natl Acad Sci 99:15440–15445
Lu L-Y, Wood JL, Ye L, Minter-Dykhouse K, Saunders TL, Yu X, Chen J (2008) Aurora A is essential for early embryonic development and tumor suppression. J Biol Chem 283:31785–31790
Ma HT, Poon RY (2020) Aurora kinases and DNA damage response. Mutation Res/Fund Mole Mech Mutagenesis 821:111716
Maitland ML, Piha-Paul S, Falchook G, Kurzrock R, Nguyen L, Janisch L, Karovic S, McKee M, Hoening E, Wong S (2018) Clinical pharmacodynamic/exposure characterisation of the multikinase inhibitor ilorasertib (ABT-348) in a phase 1 dose-escalation trial. Br J Cancer 118:1042–1050
Mallm J-P, Rippe K (2015) Aurora kinase B regulates telomerase activity via a centromeric RNA in stem cells. Cell Rep 11:1667–1678
McNeish I, Anthoney A, Loadman P, Berney D, Joel S, Halford SE, Buxton E, Race A, Ikram M, Scarsbrook A (2013) A phase I pharmacokinetic (PK) and pharmacodynamic (PD) study of the selective aurora kinase inhibitor GSK1070916A. Am Soc Clin Oncol 31:2525
Mountzios G, Terpos E, Dimopoulos M-A (2008) Aurora kinases as targets for cancer therapy. Cancer Treat Rev 34:175–182
Naz F, Anjum F, Islam A, Ahmad F, Hassan MI (2013) Microtubule affinity-regulating kinase 4: structure, function, and regulation. Cell Biochem Biophys 67:485–499
Naz H, Islam A, Ahmad F, Hassan MI (2016) Calcium/calmodulin-dependent protein kinase IV: a multifunctional enzyme and potential therapeutic target. Prog Biophys Mol Biol 121:54–65
Ota T, Suto S, Katayama H, Han Z-B, Suzuki F, Maeda M, Tanino M, Terada Y, Tatsuka M (2002) Increased mitotic phosphorylation of histone H3 attributable to AIM-1/Aurora-B overexpression contributes to chromosome number instability. Can Res 62:5168–5177
Pannone G, Hindi S, Santoro A, Sanguedolce F, Rubini C, Cincione RI, De Maria S, Tortorella S, Rocchetti R, Cagiano S (2011) Aurora B expression as a prognostic indicator and possibile therapeutic target in oral squamous cell carcinoma. Int J Immunopathol Pharmacol 24:79–88
Petsalaki E, Zachos G (2016) Clks 1, 2 and 4 prevent chromatin breakage by regulating the Aurora B-dependent abscission checkpoint. Nat Commun 7:1–13
Petsalaki E, Zachos G (2019) Building bridges between chromosomes: novel insights into the abscission checkpoint. Cell Mol Life Sci 76:4291–4307
Portella G, Passaro C, Chieffi P (2011) Aurora B: a new prognostic marker and therapeutic target in cancer. Curr Med Chem 18:482–496
Schöffski P, Jones SF, Dumez H, Infante JR, Van Mieghem E, Fowst C, Gerletti P, Xu H, Jakubczak JL, English PA (2011) Phase I, open-label, multicentre, dose-escalation, pharmacokinetic and pharmacodynamic trial of the oral aurora kinase inhibitor PF-03814735 in advanced solid tumours. Eur J Cancer 47:2256–2264
Sells TB, Chau R, Ecsedy JA, Gershman RE, Hoar K, Huck J, Janowick DA, Kadambi VJ, LeRoy PJ, Stirling M (2015) MLN8054 and Alisertib (MLN8237): discovery of selective oral Aurora A inhibitors. ACS Med Chem Lett 6:630–634
Shaalan AK, Teshima THN, Tucker AS, Proctor GB (2021) Inhibition of Aurora Kinase B activity disrupts development and differentiation of salivary glands. Cell Death Discov 7:020–00393
Shakeel I, Basheer N, Hasan GM, Afzal M, Hassan MI (2021) Polo-like Kinase 1 as an emerging drug target: structure, function and therapeutic implications. J Drug Target 29:168–184
Smith S, Bowers N, Betticher D, Gautschi O, Ratschiller D, Hoban P, Booton R, Santibanez-Koref M, Heighway J (2005) Overexpression of aurora B kinase (AURKB) in primary non-small cell lung carcinoma is frequent, generally driven from one allele, and correlates with the level of genetic instability. Br J Cancer 93:719–729
Sorrentino R, Libertini S, Pallante PL, Troncone G, Palombini L, Bavetsias V, Spalletti-Cernia D, Laccetti P, Linardopoulos S, Chieffi P (2005) Aurora B overexpression associates with the thyroid carcinoma undifferentiated phenotype and is required for thyroid carcinoma cell proliferation. J Clin Endocrinol Metab 90:928–935
Steeghs N, Eskens FA, Gelderblom H, Verweij J, Nortier JW, Ouwerkerk J, van Noort C, Mariani M, Spinelli R, Carpinelli P (2009) Phase I pharmacokinetic and pharmacodynamic study of the aurora kinase inhibitor danusertib in patients with advanced or metastatic solid tumors. J Clin Oncol 27:5094–5101
Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G (2004) A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci 101:6062–6067
Sun Y, Yang L, Hao X, Liu Y, Zhang J, Ning Z, Shi Y (2019) Phase I dose-escalation study of chiauranib, a novel angiogenic, mitotic, and chronic inflammation inhibitor, in patients with advanced solid tumors. J Hematol Oncol 12:1–10
Swedlow JR, Hirano T (2003) The making of the mitotic chromosome: modern insights into classical questions. Mol Cell 11:557–569
Tanaka M, Ueda A, Kanamori H, Ideguchi H, Yang J, Kitajima S, Ishigatsubo Y (2002) Cell-cycle-dependent regulation of human aurora A transcription is mediated by periodic repression of E4TF1. J Biol Chem 277:10719–10726
Tang C-JC, Lin C-Y, Tang TK (2006) Dynamic localization and functional implications of Aurora-C kinase during male mouse meiosis. Dev Biol 290:398–410
Tomczak K, Czerwińska P, Wiznerowicz M (2015) The cancer genome Atlas (TCGA): an immeasurable source of knowledge. Contemp Oncol 19:A68
Vader G, Medema RH, Lens SM (2006) The chromosomal passenger complex: guiding Aurora-B through mitosis. J Cell Biol 173:833–837
Wang W, Yang S, Lin J, Zeng Z, Liu D, Liu H (2009) Expression of Aurora-B in non-small cell lung cancer and its clinical significance. J Southern Med Univ 29:1853–1856
Wilkinson RW, Odedra R, Heaton SP, Wedge SR, Keen NJ, Crafter C, Foster JR, Brady MC, Bigley A, Brown E (2007) AZD1152, a selective inhibitor of Aurora B kinase, inhibits human tumor xenograft growth by inducing apoptosis. Clin Cancer Res 13:3682–3688
Willems E, Dedobbeleer M, Digregorio M, Lombard A, Lumapat PN, Rogister B (2018) The functional diversity of Aurora kinases: a comprehensive review. Cell Div 13:1–17
Wimbish RT, DeLuca KF, Mick JE, Himes J, Jiménez-Sánchez I, Jeyaprakash AA, DeLuca JG (2020) The Hec1/Ndc80 tail domain is required for force generation at kinetochores, but is dispensable for kinetochore–microtubule attachment formation and Ska complex recruitment. Mol Biol Cell 31:1453–1473
Wu X, Liu J-M, Song H-H, Yang Q-K, Ying H, Tong W-L, Zhou Y, Liu Z-L (2020) Aurora-B knockdown inhibits osteosarcoma metastasis by inducing autophagy via the mTOR/ULK1 pathway. Cancer Cell Int 20:1–14
Xie F, Zhu H, Zhang H, Lang Q, Tang L, Huang Q, Yu L (2015) In vitro and in vivo characterization of a benzofuran derivative, a potential anticancer agent, as a novel Aurora B kinase inhibitor. Eur J Med Chem 89:310–319
Yasui Y, Urano T, Kawajiri A, Nagata K-I, Tatsuka M, Saya H, Furukawa K, Takahashi T, Izawa I, Inagaki M (2004) Autophosphorylation of a newly identified site of Aurora-B is indispensable for cytokinesis. J Biol Chem 279:12997–13003
Yi Z-Y, Ma X-S, Liang Q-X, Zhang T, Xu Z-Y, Meng T-G, Ouyang Y-C, Hou Y, Schatten H, Sun Q-Y (2016) Kif2a regulates spindle organization and cell cycle progression in meiotic oocytes. Sci Rep 6:1–12
Yu J, Zhou J, Xu F, Bai W, Zhang W (2018) High expression of Aurora-B is correlated with poor prognosis and drug resistance in non-small cell lung cancer. Int J Biol Markers 33:215–221
Zekri A, Lesan V, Ghaffari SH, Tabrizi MH, Modarressi MH (2012) Gene amplification and overexpression of Aurora-C in breast and prostate cancer cell lines. Oncol Res Featuring Preclin Clin Cancer Therapeutics 20:241–250
Zhang Y, Jiang C, Li H, Lv F, Li X, Qian X, Fu L, Xu B, Guo X (2015) Elevated Aurora B expression contributes to chemoresistance and poor prognosis in breast cancer. Int J Clin Exp Pathol 8:751
Zhang X-J, Xu Y, Mou H-X, Wang S, Hao S-Y, Chen S-W (2020) The synthesis and anti-tumour properties of novel 4-substituted phthalazinones as Aurora B kinase inhibitors. Bioorganic Med Chem Lett 30:127556
Zheng YG, Wang JA, Meng L, Pei X, Zhang L, An L, Li CL, Miao YL (2021) Design, synthesis, biological activity evaluation of 3-(4-phenyl-1H-imidazol-2-yl)-1H-pyrazole derivatives as potent JAK 2/3 and aurora A/B kinases multi-targeted inhibitors. Eur J Med Chem 209:21
Acknowledgements
M.I.H. extends sincere thanks to the Council of Scientific and Industrial Research, India (Project No. 27(0368)/20/EMR-II).
Funding
Funding has been provided by Indian Council of Medical Research for financial support (Grant No. ISRM/12(22)/2020).
Author information
Authors and Affiliations
Contributions
Conceptualization: AA, AS, and MIH; writing—original draft: AA, AS, TM, and AI; writing—review and editing: AS, GMH, and MIH. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no potential conflicts of interest.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmed, A., Shamsi, A., Mohammad, T. et al. Aurora B kinase: a potential drug target for cancer therapy. J Cancer Res Clin Oncol 147, 2187–2198 (2021). https://doi.org/10.1007/s00432-021-03669-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03669-5