Abstract
Purpose
Apatinib, an antiangiogenic drug, has shown beneficial effects only in a fraction of advanced gastric cancer (GC) patients. Given the recent success of immunotherapies, combination of apatinib with immune checkpoint inhibitor may provide sustained and potent antitumor responses.
Methods
Immunocompetent mice with subcutaneous MFC tumors grown were given a combination of apatinib and anti-PD-L1 antibody therapy. GC tissues from patients undergoing curative resection in China were collected, and the density of HEVs, MSI status and tumor-infiltrated lymphocytes were analyzed by immunohistochemical staining.
Results
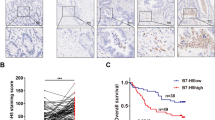
Combined apatinib and PD-L1 blockade therapy synergistically delayed tumor growth and increased survival in MFC-bearing immunocompetent mice. The combination therapy promoted antitumor immunity by increasing the ratio of CD8+ cytotoxic T cells to Foxp3+ Treg cells, the accumulation of CD20+ B cells and the Th1/Th2 cytokine ratio (IFN-γ/IL-10). The combination therapy induced the formation of HEVs through activation of LTβR signaling, thus promoting CD8+ cytotoxic T cell and CD20+ B cell infiltration in tumors. In clinical GC samples, the density of HEVs positively correlated with the intratumoral infiltration of CD8+ cytotoxic T cells and CD20+ B cells. MSI-high GC showed a higher density of HEVs, CD8+ cytotoxic T cells and CD20+ B cells than MSS/MSI-low GC. GC patients with high densities of HEVs, CD8+ cytotoxic T cells and CD20+ B cells had an improved prognosis with superior overall survival.
Conclusion
Combining apatinib with PD-L1 blockade treatment synergistically enhances antitumor immune responses and promotes HEV formation in GC.






Similar content being viewed by others
Data availability
The datasets supporting the conclusions of this article are included within the article and its additional files.
Abbreviations
- GC:
-
Gastric cancer
- HEVs:
-
High endothelial venules
- MSI:
-
Microsatellite instability
- MSS:
-
Microsatellite stable
- PD-L1:
-
Programmed death ligand 1
- VEGFR2:
-
Vascular endothelial growth factor receptor 2
- OS:
-
Overall survival
References
Allen E, Jabouille A, Rivera LB et al (2017) Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aak9679
Avram G, Sanchez-Sendra B, Martin JM et al (2013) The density and type of MECA-79-positive high endothelial venules correlate with lymphocytic infiltration and tumour regression in primary cutaneous melanoma. Histopathology 63:852–861. https://doi.org/10.1111/his.12235
Bento DC, Jones E, Junaid S et al (2015) High endothelial venules are rare in colorectal cancers but accumulate in extra-tumoral areas with disease progression. Oncoimmunology 4:e974374. https://doi.org/10.4161/2162402X.2014.974374
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Cabrita R, Lauss M, Sanna A et al (2020) Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577:561–565. https://doi.org/10.1038/s41586-019-1914-8
Chen Y, Ramjiawan RR, Reiberger T et al (2015) CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology 61:1591–1602. https://doi.org/10.1002/hep.27665
Cortes-Ciriano I, Lee S, Park WY et al (2017) A molecular portrait of microsatellite instability across multiple cancers. Nat Commun 8:15180. https://doi.org/10.1038/ncomms15180
Eso Y, Shimizu T, Takeda H et al (2020) Microsatellite instability and immune checkpoint inhibitors: toward precision medicine against gastrointestinal and hepatobiliary cancers. J Gastroenterol 55:15–26. https://doi.org/10.1007/s00535-019-01620-7
Fridman WH, Zitvogel L, Sautes-Fridman C et al (2017) The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol 14:717–734. https://doi.org/10.1038/nrclinonc.2017.101
Geng R, Song L, Li J et al (2018) The safety of apatinib for the treatment of gastric cancer. Expert Opin Drug Saf 17:1145–1150. https://doi.org/10.1080/14740338.2018.1535592
Gibney GT, Weiner LM, Atkins MB (2016) Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 17:e542–e551. https://doi.org/10.1016/S1470-2045(16)30406-5
Girard JP, Moussion C, Forster R (2012) HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol 12:762–773. https://doi.org/10.1038/nri3298
Helmink BA, Reddy SM, Gao J et al (2020) B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577:549–555. https://doi.org/10.1038/s41586-019-1922-8
Herbst RS, Arkenau HT, Santana-Davila R et al (2019) Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): a multicohort, non-randomised, open-label, phase 1a/b trial. Lancet Oncol 20:1109–1123. https://doi.org/10.1016/S1470-2045(19)30458-9
Hill DG, Yu L, Gao H et al (2018) Hyperactive gp130/STAT3-driven gastric tumourigenesis promotes submucosal tertiary lymphoid structure development. Int J Cancer 143:167–178. https://doi.org/10.1002/ijc.31298
Huang Y, Yuan J, Righi E et al (2012) Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci USA 109:17561–17566. https://doi.org/10.1073/pnas.1215397109
Jayson GC, Kerbel R, Ellis LM et al (2016) Antiangiogenic therapy in oncology: current status and future directions. Lancet 388:518–529. https://doi.org/10.1016/S0140-6736(15)01088-0
Kandalaft LE, Motz GT, Busch J et al (2011) Angiogenesis and the tumor vasculature as antitumor immune modulators: the role of vascular endothelial growth factor and endothelin. Curr Top Microbiol Immunol 344:129–148. https://doi.org/10.1007/82_2010_95
Le DT, Durham JN, Smith KN et al (2017) Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357:409–413. https://doi.org/10.1126/science.aan6733
Lee SJ, Jang BC, Lee SW et al (2006) Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7–H1 (CD274). FEBS Lett 580:755–762. https://doi.org/10.1016/j.febslet.2005.12.093
Liu XD, Hoang A, Zhou L et al (2015) Resistance to antiangiogenic therapy is associated with an immunosuppressive tumor microenvironment in metastatic renal cell carcinoma. Cancer Immunol Res 3:1017–1029. https://doi.org/10.1158/2326-6066.CIR-14-0244
Mahmoud SM, Paish EC, Powe DG et al (2011) Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 29:1949–1955. https://doi.org/10.1200/JCO.2010.30.5037
Martinet L, Garrido I, Filleron T et al (2011) Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res 71:5678–5687. https://doi.org/10.1158/0008-5472.CAN-11-0431
Martinet L, Filleron T, Le Guellec S et al (2013) High endothelial venule blood vessels for tumor-infiltrating lymphocytes are associated with lymphotoxin beta-producing dendritic cells in human breast cancer. J Immunol 191:2001–2008. https://doi.org/10.4049/jimmunol.1300872
Meder L, Schuldt P, Thelen M et al (2018) Combined VEGF and PD-L1 blockade displays synergistic treatment effects in an autochthonous mouse model of small cell lung cancer. Cancer Res 78:4270–4281. https://doi.org/10.1158/0008-5472.CAN-17-2176
Moussion C, Girard JP (2011) Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature 479:542–546. https://doi.org/10.1038/nature10540
Noman MZ, Desantis G, Janji B et al (2014) PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 211:781–790. https://doi.org/10.1084/jem.20131916
Petitprez F, de Reynies A, Keung EZ et al (2020) B cells are associated with survival and immunotherapy response in sarcoma. Nature 577:556–560. https://doi.org/10.1038/s41586-019-1906-8
Pfuderer PL, Ballhausen A, Seidler F et al (2019) High endothelial venules are associated with microsatellite instability, hereditary background and immune evasion in colorectal cancer. Br J Cancer 121:395–404. https://doi.org/10.1038/s41416-019-0514-6
Pitt JM, Vetizou M, Daillere R et al (2016) Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity 44:1255–1269. https://doi.org/10.1016/j.immuni.2016.06.001
Ramjiawan RR, Griffioen AW, Duda DG (2017) Anti-angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy? Angiogenesis 20:185–204. https://doi.org/10.1007/s10456-017-9552-y
Rosen SD (2004) Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol 22:129–156. https://doi.org/10.1146/annurev.immunol.21.090501.080131
Russo AE, Strong VE (2019) Gastric cancer etiology and management in Asia and the West. Annu Rev Med 70:353–367. https://doi.org/10.1146/annurev-med-081117-043436
Samarajiwa SA, Forster S, Auchettl K et al (2009) INTERFEROME: the database of interferon regulated genes. Nucleic Acids Res 37:D852–D857. https://doi.org/10.1093/nar/gkn732
Sato J, Kitano S, Motoi N et al (2020) CD20 (+) tumor-infiltrating immune cells and CD204 (+) M2 macrophages are associated with prognosis in thymic carcinoma. Cancer Sci 111:1921–1932. https://doi.org/10.1111/cas.14409
Sautes-Fridman C, Petitprez F, Calderaro J et al (2019) Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer 19:307–325. https://doi.org/10.1038/s41568-019-0144-6
Schmittnaegel M, Rigamonti N, Kadioglu E et al (2017) Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aak9670
Shigeta K, Datta M, Hato T et al (2020) Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology 71:1247–1261. https://doi.org/10.1002/hep.30889
von Andrian UH, Mempel TR (2003) Homing and cellular traffic in lymph nodes. Nat Rev Immunol 3:867–878. https://doi.org/10.1038/nri1222
Wculek SK, Cueto FJ, Mujal AM et al (2020) Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol 20:7–24. https://doi.org/10.1038/s41577-019-0210-z
Wirsing AM, Ervik IK, Seppola M et al (2018) Presence of high-endothelial venules correlates with a favorable immune microenvironment in oral squamous cell carcinoma. Mod Pathol 31:910–922. https://doi.org/10.1038/s41379-018-0019-5
Yi M, Jiao D, Qin S et al (2019) Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer 18:60. https://doi.org/10.1186/s12943-019-0974-6
Acknowledgements
This work was supported by the following: National Natural Science Foundation of China (81902392); Clinical Research Plan of SHDC (No. SHDC2020CR3033B); Shanghai Committee of Science and Technology (18140900502).
Funding
This work was supported by the following: National Natural Science Foundation of China (81902392); Clinical Research Plan of SHDC (No. SHDC2020CR3033B); Shanghai Committee of Science and Technology (18140900502).
Author information
Authors and Affiliations
Contributions
YZ, HRS, FW performed the experiments; YZ analyzed the data and prepared the manuscript; YZ, JPG, YKH and HH provided the samples; HRS, FW, JPG and YKH aided the data analysis. YZ and HH wrote the paper; HRS and FW commented on the study and revised the paper; HH supervised the project; YZ and HH designed the research. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
The study was approved by the ethics committee of the Fudan University Shanghai Cancer Center and signed informed consent was obtained from each patient. All animal procedures were performed according to national guidelines and approved by the Institutional Committee of Fudan University for Animal Research.
Consent to participate
Informed consent was obtained from all participants included in this study.
Consent for publication
All authors have consented to publication of the results presented in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Y., Wang, F., Sun, Hr. et al. Apatinib combined with PD-L1 blockade synergistically enhances antitumor immune responses and promotes HEV formation in gastric cancer. J Cancer Res Clin Oncol 147, 2209–2222 (2021). https://doi.org/10.1007/s00432-021-03633-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03633-3