Abstract
Purpose
The study aimed to evaluate the clinicopathological and molecular profiles associated with programmed death ligand 1 (PD-L1) expression in non-small cell lung cell (NSCLC) in a large-scale, multi-center, real-world Chinese cohort.
Methods
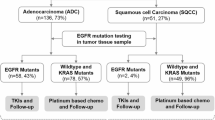
A total of 6295 NSCLC specimens from six centers in China were analyzed by PD-L1 (22C3) assay. PD-L1 expression in tumor cells (TCs) was classified as negative (TPS expression in < 1% of TCs), low (TPS in 1–49% of TCs), or high (TPS in ≥ 50% of TCs). The status of EGFR mutation was determined by reverse transcription polymerase chain reaction or next-generation sequencing, and ALK and ROS1 translocation was analyzed by immunohistochemistry and fluorescence in situ hybridization. Associations of PD-L1 expression with clinicopathological features and driver mutations were analyzed.
Results
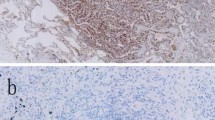
Positive PD-L1 expression was more frequently seen in squamous cell carcinoma (SCC) and other histological types of NSCLC compared to adenocarcinoma (AC). In AC, PD-L1 expression was associated with gender, histological type, metastatic status, and pathological features of lymphovascular invasion and visceral pleural invasion. Solid and micropapillary subtypes of AC were more likely to have positive PD-L1 expression compared to other subtypes. PD-L1 was more highly expressed in biopsy samples than in resected samples, and in metastatic samples compared with primary tissues. PD-L1 expression was significantly associated with wild-type EGFR and ALK translocations.
Conclusions
PD-L1 expression in NSCLC is linked to histological type, pathological features, and driver mutation status, which has meaningful implications for clinical practice.




Similar content being viewed by others
Availability of data and materials
All data relevant to the study are included in the article and uploaded as supplementary information.
References
Aredo JV, Padda SK, Kunder CA, Han SS, Neal JW, Shrager JB, Wakelee HA (2019) Impact of KRAS mutation subtype and concurrent pathogenic mutations on non-small cell lung cancer outcomes. Lung Cancer 133:144–150
Bylicki O, Paleiron N, Margery J, Guisier F, Vergnenegre A, Robinet G, Auliac JB, Gervais R, Chouaid C (2017) Targeting the PD-1/PD-L1 immune checkpoint In EGFR-mutated or ALK-translocated non-small-cell lung cancer. Target Oncol 12:563–569
Cha MJ, Lee HY, Lee KS, Jeong JY, Han J, Shim YM, Hwang HS (2014) Micropapillary and solid subtypes of invasive lung adenocarcinoma: clinical predictors of histopathology and outcome. J Thorac Cardiovasc Surg 147:921-928.e922
Chen Q, Fu YY, Yue QN, Wu Q, Tang Y, Wang WY, Wang YS, Jiang LL (2019) Distribution of PD-L1 expression and its relationship with clinicopathological variables: an audit from 1071 cases of surgically resected non-small cell lung cancer. Int J Clin Exp Pathol 12:774–786
Davis AA, Patel VG (2019) The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer 7:278
Detterbeck FC, Chansky K, Groome P, Bolejack V, Crowley J, Shemanski L, Kennedy C, Krasnik M, Peake M, Rami-Porta R (2016) The IASLC Lung Cancer Staging Project: methodology and validation used in the development of proposals for revision of the stage classification of NSCLC in the forthcoming (Eighth) edition of the TNM Classification of Lung Cancer. J Thorac Oncol 11:1433–1446
Evans M, O'Sullivan B, Hughes F, Mullis T, Smith M, Trim N, Taniere P (2018) The Clinicopathological and Molecular Associations of PD-L1 expression in non-small cell lung cancer: analysis of a series of 10,005 cases tested with the 22C3 assay. Pathol Oncol Res
Garassino MC, Gadgeel S, Esteban E et al (2020) Patient-reported outcomes following pembrolizumab or placebo plus pemetrexed and platinum in patients with previously untreated, metastatic, non-squamous non-small-cell lung cancer (KEYNOTE-189): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 21:387–397
Gradecki SE, Grange JS, Stelow EB (2018) Concordance of PD-L1 expression between core biopsy and resection specimens of non-small cell lung cancer. Am J Surg Pathol 42:1090–1094
Hirsch FR, McElhinny A, Stanforth D et al (2017) PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol 12:208–222
Hong S, Chen N, Fang W et al (2016) Upregulation of PD-L1 by EML4-ALK fusion protein mediates the immune escape in ALK positive NSCLC: implication for optional anti-PD-1/PD-L1 immune therapy for ALK-TKIs sensitive and resistant NSCLC patients. Oncoimmunology 5:e1094598
Huynh TG, Morales-Oyarvide V, Campo MJ et al (2016) Programmed cell death ligand 1 expression in resected lung adenocarcinomas: association with immune microenvironment. J Thorac Oncol 11:1869–1878
Jain P, Jain C, Velcheti V (2018) Role of immune-checkpoint inhibitors in lung cancer. Ther Adv Respir Dis 12:1753465817750075
Jin Y, Shen X, Pan Y, Zheng Q, Chen H, Hu H, Li Y (2019) Correlation between PD-L1 expression and clinicopathological characteristics of non-small cell lung cancer: a real-world study of a large Chinese cohort. J Thorac Dis 11:4591–4601
Mok TSK, Wu YL, Kudaba I et al (2019) Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 393:1819–1830
Morales-Oyarvide V, Mino-Kenudson M (2014) High-grade lung adenocarcinomas with micropapillary and/or solid patterns: a review. Curr Opin Pulm Med 20:317–323
Munari E, Rossi G, Zamboni G et al (2018) PD-L1 Assays 22C3 and SP263 are not interchangeable in non-small cell lung cancer when considering clinically relevant cutoffs: an interclone evaluation by differently trained pathologists. Am J Surg Pathol 42:1384–1389
Munari E, Zamboni G, Lunardi G et al (2018) PD-L1 expression heterogeneity in non-small cell lung cancer: defining criteria for harmonization between biopsy specimens and whole sections. J Thorac Oncol 13:1113–1120
Naso JR, Wang G, Pender A, Wong SK, Zhu J, Ho C, Ionescu DN, Zhou C (2020) Intratumoral heterogeneity in programmed death-ligand 1 immunoreactivity is associated with variation in non-small cell lung carcinoma histotype. Histopathology 76:394–403
Pan Y, Zheng D, Li Y et al (2017) Unique distribution of programmed death ligand 1 (PD-L1) expression in East Asian non-small cell lung cancer. J Thorac Dis 9:2579–2586
Rittmeyer A, Barlesi F, Waterkamp D et al (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389:255–265
Rizvi H, Sanchez-Vega F, La K et al (2018) Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol 36:633–641
Saito Y, Horiuchi S, Morooka H, Ibi T, Takahashi N, Ikeya T, Shimizu Y, Hoshi E (2019) Inter-tumor heterogeneity of PD-L1 expression in non-small cell lung cancer. J Thorac Dis 11:4982–4991
Sun JM, Zhou W, Choi YL et al (2016) Prognostic significance of PD-L1 in patients with non-small cell lung cancer: a large cohort study of surgically resected cases. J Thorac Oncol 11:1003–1011
Tsao MS, Kerr KM, Kockx M et al (2018) PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. J Thorac Oncol 13:1302–1311
Tseng JS, Yang TY, Wu CY, Ku WH, Chen KC, Hsu KH, Huang YH, Su KY, Yu SL, Chang GC (2018) Characteristics and predictive value of PD-L1 status in real-world non-small cell lung cancer patients. J Immunother 41:292–299
Xu H, Li X, Adams H, Kubena K, Guo S (2018) Etiology of metabolic syndrome and dietary intervention. Int J Mol Sci 20
Xu H, Chen X, Lin D, Zhang J, Li C, Zhang D, Zhang X (2019) Conformance assessment of PD-L1 expression between primary tumour and nodal metastases in non-small-cell lung cancer. Onco Targets Ther 12:11541–11547
Yoneshima Y, Ijichi K, Anai S, Ota K, Otsubo K, Iwama E, Tanaka K, Oda Y, Nakanishi Y, Okamoto I (2018) PD-L1 expression in lung adenocarcinoma harboring EGFR mutations or ALK rearrangements. Lung Cancer 118:36–40
Acknowledgments
Editorial assistance and proofreading were provided by Medjaden Bioscience Limited. Funding for this assistance service was provided by MSD China.
Funding
This study was supported by National Nature Science Foundation of China grant number 81972171 and 81472173.
Author information
Authors and Affiliations
Contributions
XC, CW, YL conceived, designed and planned the study. YH, XZ, XC, QX, YW, YG and BG provided study materials and patients. CW provided administrative and technical support. QZ, YH, XC, XZ, SS, YJ, QX, YW, YG and BG collected the data. QZ and YH performed analyses. YW performed statistical expertise. QZ and CW interpreted the results. QZ, XC, QX, YW and YL wrote the initial draft. QZ, YH, XZ, SS, CW, YJ, QX, YG, BG and YL provided suggestions for revision of the manuscript. All authors reviewed and approved final version of the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
The study was approved by the Institutional Review Board of Fudan University Shanghai Cancer Center. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for publication
All data published here are under the consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zheng, Q., Huang, Y., Zeng, X. et al. Clinicopathological and molecular characteristics associated with PD-L1 expression in non-small cell lung cancer: a large-scale, multi-center, real-world study in China. J Cancer Res Clin Oncol 147, 1547–1556 (2021). https://doi.org/10.1007/s00432-020-03444-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-020-03444-y