Abstract
Purpose
Testicular granulosa cell tumors (tGrCT) are rare sex cord-stromal tumors. This review aims to synthesize the available evidence regarding the clinical presentation and clinicopathological characteristics, treatment and outcomes.
Methods
We conducted a systematic literature search using the most important research databases. Whenever feasible, we extracted the data on individual patient level.
Results
From 7863 identified records, we included 88 publications describing 239 patients with tGrCT. The majority of the cases were diagnosed with juvenile tGrCT (166/239, 69%), while 73/239 (31%) patients were diagnosed with adult tGrCT. Mean age at diagnosis was 1.5 years (± 5 SD) for juvenile tGrCT, and 42 years (± 19 SD) for adult tGrCT. Information on primary treatment was available in 231/239 (97%), of which 202/231 (87%) were treated with a radical orchiectomy and 20/231 (9%) received testis sparing surgery (TSS). Local recurrence after TSS was observed in 1/20 (5%) cases. Metastatic disease was never observed in men with juvenile tGrCT but in 7/73 (10%) men with adult tGrCT. In 5/7 men with metastatic tGrCT, metastases were diagnosed at initial staging, while 2/7 patients developed metastases after 72 and 121 months of follow-up, respectively. Primary site of metastasis is represented by the retroperitoneal lymph nodes, but other sites including lungs, liver, bone and inguinal lymph nodes can also be affected. In comparison with non-metastatic adult tGrCT, men with metastatic adult tGrCT had significantly larger primary tumors (70 vs 24 mm, p 0.001), and were more likely to present with angiolymphatic invasion (57% vs 4%, p 0.002) or gynecomastia (29% vs 3%, p 0.019). In five out of seven men with metastatic disease, resection of metastases or platinum-based chemotherapy led to complete remission.
Conclusion
Juvenile tGrCT represent a benign entity whereas adult tGCTs have metastatic potential. Tumor size, presence of angiolymphatic invasion or gynecomastia represent risk factors for metastatic disease. The published literature supports the use of testis sparing surgery but there is only limited experience with adjuvant therapies. In the metastatic setting, the reviewed literature suggests that aggressive surgical and systemic treatment might cure patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Testicular granulosa cell tumors (tGrCT) are a rare group of sex cord-stromal tumors (SCST) originating from epithelial elements of the sex cord. While they represent the most common SCST in the ovary (Young 2005), the testicular manifestation was only reported sporadically since the first description in 1952 (Laskowski 1952). According to the current World Health Organization (WHO) classification of Tumors of the Urinary System and Male Genital Organs, two histologic subtypes are distinguished: The juvenile tGrCT and the less frequent adult tGrCT (Idrees et al. 2017). While the juvenile subtype accounts for 6% of all prepubertal testicular tumors and represents the most frequent congenital testicular tumor (Kao et al. 2015), the adult tGrCT is rare and only reported in small case series and case reports. For both histological subtypes, the risk of metastatic spread is ill defined (Cecchetto et al. 2010; Mostofi et al. 1959).
Due to the rarity of tGrCT, there are several unanswered questions regarding the optimal management of patients with localized or metastatic tGrCT. The aim of this systematic literature review was to provide an overview of the available data on tGrCT patients, regarding clinical presentation, clinicopathologic factors predicting metastatic disease, experience with testis sparing surgery, sites of metastasis, and outcome and treatment success in case of metastatic disease.
Methods
Evidence acquisition
Data acquisition and search strategy
This systematic literature review was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement (Moher et al. 2009, 2015). Prior to data acquisition, the review protocol and search strategy were published on the University of York’s PROSPERO registry (http://www.crd.york.ac.uk/PROSPERO; registration number CRD42018110112).
Our literature search identified articles published up to 5th May 2018 and covered the most significant electronic databases (MEDLINE, EMBASE, Scopus, Cochrane Database of Systematic Reviews and Web of Science). A clinical medical librarian applied a broad approach using several combinations, synonyms and search terms related to “granulosa cell tumor”, “sex cord tumor”, “stromal tumor” or “interstitial cell tumor” to identify all relevant articles. Non-English literature was excluded unless the abstract was available in English or the full text in French, Spanish, Italian or German. The reference lists of the identified publications were screened manually to identify additional studies. A detailed description of our search strategy is shown in Appendix 1.
Deduplication of the resulting list of publications was achieved automatically using the close match function of our reference management software. The remaining duplicate articles were identified via manual deduplication done by two authors (JG, KS). The same authors screened the titles and abstracts independently to select publications that fulfilled the eligibility criteria and came to a consensus about the inclusion of those studies. Data of the same study that appeared in multiple publications were counted only once in the synthesis. Disagreements were discussed and resolved by consensus or by third-party arbitration (CDF). Any case reports, clinical case series and other reports describing patients with juvenile or adult tGrCT were included.
Types of outcome measures included
Studies reporting clinical or pathological variables, treatment of local or metastatic disease, site of metastases, disease-free, cancer-specific or overall survival were eligible for this review. To capture all relevant literature, our search strategy did not include predefined interventions, controls or outcomes.
Data extraction
Based on the Cochrane Consumers and Communication Review Group’s data extraction template, a data extraction sheet was developed and improved after a pilot-testing phase on twenty randomly selected eligible studies. Data on study design, patient characteristics, clinicopathological risk factors, treatment and follow-up were collected. Whenever feasible, data were gathered on single patient level.
Statistical analysis
Receiver operating curve (ROC) analyses using the maximal Youden’s index (= Sensitivity + Specificity − 1) (Youden 1950) were used to determine an optimal cut-off value of continuous variables. 2 × 2 table analysis was performed with R version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria) using the package epiR. Descriptive data are presented as median and interquartile range (IQR). Weighted medians were used to estimate already statistically processed data of cohort studies as well as individual patient data of single case reports. The results for continuous normally distributed variables are expressed as mean ± standard deviation (SD). Continuous non-normally distributed variables are presented as median and interquartile ranges (IQR) and categorical variables are presented as percentage. Categorical data were compared using the Chi-square test of independence. Normally distributed continuous variables were analyzed using the independent samples t test, while the Mann–Whitney U test was used for non-normally distributed continuous variables. All p values < 0.05 were considered statistically significant. All statistical tests were two sided.
Evidence synthesis
Studies
After automated and manual deduplication, 98 of 3542 publications which met the initial search criteria were eligible for full-text review after screening of title and abstract. We finally included 88 studies, resulting in a dataset of 239 patients (Fig. 1). Data regarding follow-up were available in 185 of 239 (77%) patients. Mean follow-up time was 48 months (± 40 SD).
Subgroups, demographics, clinical symptoms and laboratory findings
We identified 239 cases of tGrCT, of which 166 (69%) presented with the histological variant juvenile tGrCT and 73 (31%) with adult tGrCT (Table 1). Juvenile tGrCT were diagnosed at a mean age of 1.5 years (± 5 SD), with single reports of diagnosis in early adulthood (27 and 34 years) (Gravas et al. 2007; Lin et al. 2008). Patients with adult tGrCT were diagnosed at a mean age of 42 years (± 19 SD). Overall, data about clinical presentation were available in 171 of the 239 patients (72%). The majority of patients presented with a testicular mass (78/171, 46%) or testicular enlargement (71/171, 42%), whereas scrotal pain was only described in 8/171 (5%) cases. Alpha-fetoprotein (AFP) was elevated in 18/171 (11%) of the cases, whereas other tumor markers like human chorionic gonadotropin (HCG) and lactate dehydrogenase (LDH) were elevated in only 3/171 (2%) and 2/171 (1%) patients, respectively. Hormonal changes were reported in 19/171 (11%), including gynecomastia (7/171, 4%) and anomaly of puberty (2/171, 1%). History of ipsi- or contralateral cryptorchidism was reported in 18/166 (11%) of juvenile tGrCT and in 3/73 (4%) of adult tGrCT.
Local treatment and pathological findings
In 231 of 239 cases, information about treatment was available (Table 2). Most patients underwent orchiectomy (202/231, 87%), while testis sparing surgery (TSS) was performed in 20 cases (9%). Local recurrence 3 months after TSS was reported in one patient (1/20, 5%). After receiving a salvage hemiscrotectomy, the patient remained disease free for at least 18 months (Gravas et al. 2007). Seven patients (3%) with intra-abdominal cryptorchidism underwent laparotomy. Two patients (1%) underwent testicular biopsy only without any further treatment. The median tumor size of tGCTs was 21 mm (IQR 15–44). The most frequently described pathological findings included high mitotic rate (53/239, 22%), necrosis (12/239, 5%), angiolymphatic invasion (10/239, 4%) and pleomorphism (10/239, 4%). Other features like infiltrating margins, extracapsular invasion or calcification were seen in four patients or less. The most frequently reported immunohistochemistry included vimentin (58/239, 24%), inhibin (55/239, 23%) and calretinin (24/239, 10%).
Metastatic disease
While all of the 166 reported cases of juvenile tGrCT were exclusively benign, 7 out of 73 (10%) patients with adult tGrCT showed metastatic disease (Table 3). Mean age at diagnosis of patients with metastatic tGrCT was 45 years (± 14 SD). The median primary tumor size of metastatic cases was 70 mm (IQR: 51–90). Painless testicular enlargement (n = 3) or palpable mass (n = 2) was the most frequent clinical presentation in metastatic tGCTs, two patients also presented with gynecomastia. One patient was diagnosed incidentally during an ultrasound examination. None of the reported metastatic cases showed elevated AFP, HCG or LDH.
Risk factors for metastatic disease
Predictive variables for metastatic disease included tumor size, angiolymphatic invasion and presence of gynecomastia (supplementary Table 1). According to ROC analyses, the ideal cut-off for tumor size to predict metastatic disease was 46 mm (AUC 0.86 95% CI 0.76–0.93). A tumor size above 46 mm was observed in 7/7 (100%) metastatic and in (10/61, 16%, p value: < 0.001) non-metastatic patients. Angiolymphatic invasion was more common in metastatic compared to non-metastatic disease (4/7 (57%) vs. 8/54 (15%), p = 0.002). Furthermore, gynecomastia was more common in metastatic compared to non-metastatic disease (29% vs. 3%, p = 0.019).
Onset of metastatic disease
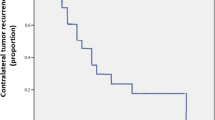
In five patients, metastatic disease was diagnosed at initial staging. The primary site of metastatic disease at staging included retroperitoneal lymph nodes (RPLN) in four and the chest in one patient (Fig. 2). Overall, three patients developed metastatic disease recurrence: Two patients with localized disease at staging showed metastatic recurrence (2/66, 3%) after a 72 and 121 months, respectively. Sites of recurrence included distal tibia in one case and the retroperitoneum and liver in the second case. One patient with metastatic disease in the retroperitoneum at initial staging showed recurrence in the inguinal lymph nodes later on (Table 4).
Treatment of metastatic disease
The median follow-up time for men with metastatic disease was 14 months (IQR: 9–83). Of seven patients with metastasized tGrCT, five patients showed complete remission after treatment (Table 4). Patient #1 had a metastatic recurrence in the distal left tibia 6 years after diagnosis and received a below knee amputation and was free of disease for at least another 4 months (Suppiah et al. 2005). Patient #2 and #3 showed metastatic disease in the RPLN at initial staging were treated with RPLND and remained without evidence of disease during a total follow-up time of 32 (Mohapatra et al. 2016) and 168 months (Matoska et al. 1992), respectively. Patient #4 with metastatic disease in the RPLN received four cycles of BEP and remained disease free for at least 6 months (Elbachiri et al. 2017). Patient #5 presented with lung metastases at initial staging, received 6 cycles of BEP and showed no evidence of disease for at least 13 months (Hammerich et al. 2008). Patient #6 was diagnosed with RPLN and liver metastases 121 months after orchiectomy. He subsequently received chemotherapy with doxorubicin–cisplatin (DC) but died of progressive disease 134 months after initial diagnosis (Matoska et al. 1992). Patient #7 underwent a modified retroperitoneal lymph node dissection, which revealed metastatic involvement of four lymph nodes. Consequently, the patient was treated with one cycle of chemotherapy with etoposide, which was discontinued because of side effects. After 2 months, ipsilateral inguinal lymph node metastases were detected. After resection and additive radiotherapy, he remained disease free for at least 14 months (Matoska et al. 1992).
Discussion
Our systematic review of published case series represents the most comprehensive summary of the available literature regarding tGCTs providing recommendation for (1) local therapy, (2) risk factors for metastatic disease and recommendations for follow-up and (3) treatment of metastatic disease.
First, based on the low local recurrence rate of 5%, the use of TSS as primary treatment can be considered in case of juvenile tGrCT and in selected cases with small adult tGrCTs. However, the evidence is limited and only based on a minority of the cases treated with TSS. Patients should be informed about the low risk of local recurrence and the requirement for a completion orchiectomy in case of angiolymphatic invasion. If TSS is to be used, follow-up with testicular ultrasound should be considered.
Second, to identify adult tGrCT with metastatic potential, a larger tumor size, presence of angiolymphatic invasion and presence of gynecomastia represent predictive variables with good discriminatory accuracy. Given the high negative predictive value of those risk and late onset of recurrences, no general recommendation for regular follow-up with cross-sectional imaging of the abdomen and chest can be given. Instead, we recommend regular follow-up examination by a uro-oncologist for patients with risk factors on an annual basis for an extended postoperative period of up to 10–15 years.
On the other hand, given the low risk of recurrence of 3%, we recommend patients without risk factors or juvenile tGrCT to be followed up by their general practitioner. Imaging and/or referral to a uro-oncologist is recommended in case of clinical suspicion. Our data do not support the use of germ-cell tumor markers such as AFP, HCG, LDH during the follow-up of tGrCT patients.
Third, the non-existence of data regarding adjuvant therapy for localized disease questions the use of any adjuvant therapies. The fact that in 80% the primary metastatic tGrCT landing site was the retroperitoneum adjuvant RPLND might have the potential to cure some patients with micro-metastatic disease. However, as our data do not provide evidence that tGrCT have a discreet step-wise progression involving a specific primary landing site, adjuvant RPLND is not recommended.
Fourth, although the experience in men with metastatic disease is limited, response to surgical resection of metastases and/or chemotherapy with BEP was observed. We, therefore, suggest an aggressive curative approach with surgery alone or in combination with cisplatin-based chemotherapy in case of incomplete resection.
Limitations
The published literature only consists of retrospective case reports and small case series. Moreover, reports of clinicopathological features were often inconsistent, making this analysis prone to bias. Our search strategy was designed and reviewed both by clinicians as well as librarians and was predefined in a peer-reviewed protocol. However, the possibility remains that not all potentially relevant studies were identified, which could be classified as an additional source of bias. Given the limited number of seven metastatic events, we refrained from running multivariable regression analyses and larger datasets are needed to develop prediction models involving several risk factors. The current analysis provides a unique overview of the published experience with juvenile and adult tGrCT. It may help physicians differentiate between tGrCT with a lower or higher risk for metastatic disease and select the most appropriate treatment modality for tGrCT patients.
Due to the absence of prospective trials, we recently opened the OrphAn Testis Histologies (OATH) to provide more conclusive recommendations regarding clinical course, management and follow-up of these rare entities. We encourage collaborators to contribute data of patients with rare testis cancer histologies (http://bit.ly/OATH-registry).
Abbreviations
- tGrCT:
-
Testicular granulosa cell tumors
- SCST:
-
Sex cord-stromal tumors
- WHO:
-
World Health Organization
- TSS:
-
Testis sparing surgery
- NOS:
-
Not otherwise specified
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analysis
- IQR:
-
Interquartile range
- SD:
-
Standard deviation
- ROC:
-
Receiver operating curve
- AFP:
-
Alpha fetoprotein
- HCG:
-
Human chorionic gonadotropin
- LDH:
-
Lactate dehydrogenase
- RPLND:
-
Retroperitoneal lymph node dissection
- BEP:
-
Bleomycin, etoposide and cisplatin
- RPLN:
-
Retroperitoneal lymph nodes
- HPF:
-
High-power field
- DC:
-
Doxorubicin–cisplatin
- OATH:
-
OrphAn Testis Histologies
References
Cecchetto G et al (2010) Sex cord-stromal tumors of the testis in children. A clinicopathologic report from the Italian TREP project. J Pediatr Surg 45:1868–1873. https://doi.org/10.1016/j.jpedsurg.2010.02.120
Elbachiri M et al (2017) Adult-type granulosa cell tumor of the testis: report of a case and review of literature. Pan Afr Med J 26:198. https://doi.org/10.11604/pamj.2017.26.198.11523
Gravas S, Georgiadis T, Vassiliadis F, Kehayas P (2007) Juvenile granulosa cell tumor of the epididymis. Urol Int 78:278–279. https://doi.org/10.1159/000099352
Hammerich KH, Hille S, Ayala GE, Wheeler TM, Engers R, Ackermann R, Mueller-Mattheis V (2008) Malignant advanced granulosa cell tumor of the adult testis: case report and review of the literature. Hum Pathol 39:701–709. https://doi.org/10.1016/j.humpath.2007.09.015
Idrees MT et al (2017) The World Health Organization 2016 classification of testicular non-germ cell tumours: a review and update from the International Society of Urological Pathology Testis Consultation Panel. Histopathology 70:513–521. https://doi.org/10.1111/his.13115
Kao CS, Cornejo KM, Ulbright TM, Young RH (2015) Juvenile granulosa cell tumors of the testis: a clinicopathologic study of 70 cases with emphasis on its wide morphologic spectrum. Am J Surg Pathol 39:1159–1169. https://doi.org/10.1097/pas.0000000000000450
Laskowski J (1952) Feminizing tumors of the testis: general review with case report of granulosa cell tumor of the testis. Endokrynol Pol 3:337–343
Lin KH, Lin SE, Lee LM (2008) Juvenile granulosa cell tumor of adult testis: a case report. Urology 72:230.e211–230.e233. https://doi.org/10.1016/j.urology.2007.11.126
Matoska J, Ondrus D, Talerman A (1992) Malignant granulosa cell tumor of the testis associated with gynecomastia and long survival. Cancer 69:1769–1772
Mohapatra A, Potretzke AM, Knight BA, Han M, Figenshau RS (2016) Metastatic granulosa cell tumor of the testis: clinical presentation and management case. Rep Urol 2016:9016728. https://doi.org/10.1155/2016/9016728
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62:1006–1012. https://doi.org/10.1016/j.jclinepi.2009.06.005
Moher D et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4:1
Mostofi FK, Theiss EA, Ashley DJ (1959) Tumors of specialized gonadal stroma in human male patients. Androblastoma, Sertoli cell tumor, granulosa-theca cell tumor of the testis, and gonadal stromal tumor. Cancer 12:944–957
Suppiah A, Musa MM, Morgan DR, North AD (2005) Adult granulosa cell tumour of the testis and bony metastasis. A report of the first case of granulosa cell tumour of the testicle metastasising to bone. Urol Int 75:91–93. https://doi.org/10.1159/000085936
Youden WJ (1950) Index for rating diagnostic tests. Cancer 3:32–35
Young RH (2005) Sex cord-stromal tumors of the ovary and testis: their similarities and differences with consideration of selected problems. Mod Pathol 18(Suppl 2):S81–S98. https://doi.org/10.1038/modpathol.3800311
Acknowledgements
Open access funding provided by University of Zurich. We would like to thank the clinical medical librarians M. Hediger and M. Gosteli for their expertise and support during both planning and execution of our systematic literature search.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grogg, J.B., Schneider, K., Bode, PK. et al. Risk factors and treatment outcomes of 239 patients with testicular granulosa cell tumors: a systematic review of published case series data. J Cancer Res Clin Oncol 146, 2829–2841 (2020). https://doi.org/10.1007/s00432-020-03326-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-020-03326-3