Abstract
Purpose
Based on an exceptionally durable response to pemetrexed observed in some patients with metastatic NSCLC, the predictive value of pemetrexed sensitivity to outcomes of subsequent systemic treatment was investigated.
Methods
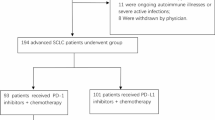
We retrospectively reviewed the patients with metastatic non-squamous NSCLC treated with pemetrexed monotherapy as their first- or second-line chemotherapy between November 2006 and February 2015. Good (top 5% longest) and poor responders (bottom 12% shortest) were defined according to the duration of pemetrexed maintenance. The first and second post-pemetrexed (PP) systemic treatments were defined as PP1 and PP2 therapies, respectively, to define their progression-free survivals (PFS) as PFS1 and PFS2.
Results
In a total of 100 patients, 86% of patients received pemetrexed as their second-line chemotherapy, and 34% were classified as good responders. Good and poor responder groups showed 20.5 months and 0.7 months of the median duration of responses, respectively. PP1 and PP2 therapies were done in 74% and 41.9% of patients after failure to pemetrexed. To our surprise, disease control rate (DCR) was significantly higher in the good responder group than poor responder group (69.6% vs 37.3%, p = 0.010) in patients treated with PP1 therapy, and median PFS1 was also significantly longer (5.2 vs 2.2 months, p < 0.01) regardless of the type of subsequent systemic treatment. Meanwhile, pemetrexed sensitivity did not affect DCR or PFS of patients who received PP2 therapies.
Conclusions
Patients who achieved durable response to pemetrexed might obtain greater therapeutic benefits from subsequent systemic treatment in metastatic non-squamous NSCLC without targets, which could potentiate more effective post-pemetrexed treatment strategy.


Similar content being viewed by others
References
Adjei AA (2004) Pharmacology and mechanism of action of pemetrexed. Clinical Lung Cancer 5(Suppl 2):S51–55
Barlesi F, Mazieres J, Merlio J-P, Debieuvre D, Mosser J, Lena H, Ouafik LH, Besse B, Rouquette I, Westeel V, Escande F, Monnet I, Lemoine A, Veillon R, Blons H, Audigier-Valette C, Bringuier P-P, Lamy R, Beau-Faller M, Pujol J-L, Sabourin J-C, Penault-Llorca F, Denis MG, Lantuejoul S, Morin F, Tran Q, Missy P, Langlais A, Milleron B, Cadranel J, Soria J-C, Zalcman G (2016) Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). The Lancet 387(10026):1415–1426. https://doi.org/10.1016/S0140-6736(16)00004-0
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR (2015) Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med 373(17):1627–1639. https://doi.org/10.1056/NEJMoa1507643
Chattopadhyay S, Moran RG, Goldman ID (2007) Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol Cancer Ther 6(2):404–417. https://doi.org/10.1158/1535-7163.Mct-06-0343
Chen K-C, Yang T-Y, Wu C-C, Cheng C-C, Hsu S-L, Hung H-W, Chen J-W, Chang G-C (2014) Pemetrexed induces s-phase arrest and apoptosis via a deregulated activation of Akt signaling pathway. PLoS ONE 9(5):e97888. https://doi.org/10.1371/journal.pone.0097888
Davis M, Conlon K, Bohac GC, Barcenas J, Leslie W, Watkins L, Lamzabi I, Deng Y, Li Y, Plate JM (2012) Effect of pemetrexed on innate immune killer cells and adaptive immune T cells in subjects with adenocarcinoma of the pancreas. J Immunother (Hagerstown Md: 1997) 35(8):629–640. https://doi.org/10.1097/cji.0b013e31826c8a4f
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 11). Eur J Cancer (Oxford, Engl: 1990) 45(2):228–247
Emens LA, Middleton G (2015) The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res 3(5):436–443. https://doi.org/10.1158/2326-6066.Cir-15-0064
Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G (2015) Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 28(6):690–714. https://doi.org/10.1016/j.ccell.2015.10.012
Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378(22):2078–2092. https://doi.org/10.1056/NEJMoa1801005
Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, Powderly JD, Heist RS, Carvajal RD, Jackman DM, Sequist LV, Smith DC, Leming P, Carbone DP, Pinder-Schenck MC, Topalian SL, Hodi FS, Sosman JA, Sznol M, McDermott DF, Pardoll DM, Sankar V, Ahlers CM, Salvati M, Wigginton JM, Hellmann MD, Kollia GD, Gupta AK, Brahmer JR (2015) Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 33(18):2004–U2032. https://doi.org/10.1200/jco.2014.58.3708
Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (Lond Engl) 387(10027):1540–1550. https://doi.org/10.1016/s0140-6736(15)01281-7
Kano Y, Akutsu M, Tsunoda S, Izumi T, Kobayashi H, Inoue K, Mori K, Fujii H, Mano H, Odgerel T, Furukawa Y (2006) Schedule-dependent interactions between pemetrexed and cisplatin in human carcinoma cell lines in vitro. Oncol Res 16(2):85–95
Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ, Asahina H, Jones RE, Kulkarni MM, Kuraguchi M, Palakurthi S, Fecci PE, Johnson BE, Janne PA, Engelman JA, Gangadharan SP, Costa DB, Freeman GJ, Bueno R, Hodi FS, Dranoff G, Wong KK, Hammerman PS (2016) Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun 7:9. https://doi.org/10.1038/ncomms10501
Kuo WT, Tu DG, Chiu LY, Sheu GT, Wu MF (2017) High pemetrexed sensitivity of docetaxel-resistant A549 cells is mediated by TP53 status and downregulated thymidylate synthase. Oncol Rep 38(5):2787–2795. https://doi.org/10.3892/or.2017.5951
Lin EP-Y, Yang C-Y, Lin C-W, Huang B-T, Lai W-Y, Tseng Y-T, Yang P-C (2017) Priming PD-L1 expression by chemotherapeutic agents in non-small cell lung cancers. J Clin Oncol 35(15_suppl):e20087–e20087. https://doi.org/10.1200/jco.2017.35.15_suppl.e20087
Maleki Vareki S, Chen D, Di Cresce C, Ferguson PJ, Figueredo R, Pampillo M, Rytelewski M, Vincent M, Min W, Zheng X, Koropatnick J (2015) IDO downregulation induces sensitivity to pemetrexed, gemcitabine, FK866, and methoxyamine in human cancer cells. PLoS ONE 10(11):e0143435. https://doi.org/10.1371/journal.pone.0143435
Mok TS, Wu Y-L, Thongprasert S, Yang C-H, Chu D-T, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang J-J, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361(10):947–957. https://doi.org/10.1056/NEJMoa0810699
Park S, Kim HJ, Choi CM, Lee DH, Kim SW, Lee JS, Kim WS, Choi SH, Rho JK, Lee JC (2016) Predictive factors for a long-term response duration in non-squamous cell lung cancer patients treated with pemetrexed. BMC cancer 16:417. https://doi.org/10.1186/s12885-016-2457-0
Paz-Ares LG, de Marinis F, Dediu M, Thomas M, Pujol JL, Bidoli P, Molinier O, Sahoo TP, Laack E, Reck M, Corral J, Melemed S, John W, Chouaki N, Zimmermann AH, Visseren-Grul C, Gridelli C (2013) PARAMOUNT: final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 31(23):2895. https://doi.org/10.1200/jco.2012.47.1102
Prendergast GC, Smith C, Thomas S, Mandik-Nayak L, Laury-Kleintop L, Metz R, Muller AJ (2014) Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother CII 63(7):721–735. https://doi.org/10.1007/s00262-014-1549-4
Putri DU, Feng P-H, Hsu Y-H, Lee K-Y, Jiang F-W, Kuo L-W, Chen Y-J, Han C-L (2018) Chemotherapy immunophenoprofiles in non-small-cell lung cancer by personalized membrane proteomics. Proteom Clin Appl 12(2):1700040. https://doi.org/10.1002/prca.201700040
Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR (2016a) Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med 375(19):1823–1833. https://doi.org/10.1056/NEJMoa1606774
Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR (2016b) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375(19):1823–1833. https://doi.org/10.1056/NEJMoa1606774
Restifo NP, Smyth MJ, Snyder A (2016) Acquired resistance to immunotherapy and future challenges. Nat Rev Cancer 16:121. https://doi.org/10.1038/nrc.2016.2
Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, Garrido P, Longo F, Moran T, Insa A, De Marinis F, Corre R, Bover I, Illiano A, Dansin E, de Castro J, Milella M, Reguart N, Altavilla G, Jimenez U, Provencio M, Moreno MA, Terrasa J, Muñoz-Langa J, Valdivia J, Isla D, Domine M, Molinier O, Mazieres J, Baize N, Garcia-Campelo R, Robinet G, Rodriguez-Abreu D, Lopez-Vivanco G, Gebbia V, Ferrera-Delgado L, Bombaron P, Bernabe R, Bearz A, Artal A, Cortesi E, Rolfo C, Sanchez-Ronco M, Drozdowskyj A, Queralt C, de Aguirre I, Ramirez JL, Sanchez JJ, Molina MA, Taron M, Paz-Ares L (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13(3):239–246. https://doi.org/10.1016/S1470-2045(11)70393-X
Tièche CC, Peng R-W, Dorn P, Froment L, Schmid RA, Marti TM (2016) Prolonged pemetrexed pretreatment augments persistence of cisplatin-induced DNA damage and eliminates resistant lung cancer stem-like cells associated with EMT. BMC Cancer 16:125–125. https://doi.org/10.1186/s12885-016-2117-4
Wilson PM, Danenberg PV, Johnston PG, Lenz H-J, Ladner RD (2014) Standing the test of time: targeting thymidylate biosynthesis in cancer therapy. Nat Rev Clin Oncol 11:282. https://doi.org/10.1038/nrclinonc.2014.51
Yoshida T, Okamoto T, Yano T, Takada K, Kohno M, Suda K, Takenoyama M, Oda Y, Maehara Y (2016) Molecular factors associated with pemetrexed sensitivity according to histological type in non-small cell lung cancer. Anticancer Res 36(12):6319–6326. https://doi.org/10.21873/anticanres.11228
Zhang P, Bao Z, Xu L, Zhou J, Lu G, Yao Y, Liu R, Gao Q, Shen Y, Zhou J (2017) PD-L1 expression indicates favorable prognosis for advanced lung adenocarcinoma patients treated with pemetrexed. Oncotarget 8(39):66293–66304. https://doi.org/10.18632/oncotarget.19973
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No author has any financial disclosures to declare related to this study.
Research involving human participants
It was conducted in full accordance with the Guidelines for Good Clinical Practice and the 1964 Declaration of Helsinki.
Informed consent
The study protocol was reviewed and approved by the Institutional Review Board (IRB Approval Number: 2018-0661) of Asan Medical Center, and informed consents were obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Park, J.H., Kwon, B.S., Park, S.J. et al. Exceptional pemetrexed sensitivity can predict therapeutic benefit from subsequent chemotherapy in metastatic non-squamous non-small cell lung cancer. J Cancer Res Clin Oncol 145, 1897–1905 (2019). https://doi.org/10.1007/s00432-019-02941-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-019-02941-z