Abstract
Purpose
To report the dosimetric feasibility of the radiation technique HALFMOON (Helical ALtered Fractionation for iMplant partial OmissiON) for post-mastectomy radiation therapy (PMRT) in intermediate–high-risk breast cancer patients with implant-based immediate breast reconstruction, where the clinical target volume (CTV) does not include the whole implant (implant-sparing approach).
Methods
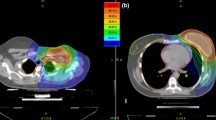
In the HALFMOON technique, the CTV consisted of skin, subcutaneous tissues, and pectoralis major muscle, excluding the implant, chest wall muscles, and rib plane. The HALFMOON plans were compared with conventionally contoured CTV plans, in which the whole implant, chest wall muscles, and ribs plane were included in the CTV, in a ratio 1:3. All patients underwent hypofractionated treatment of 40.05 Gy/15 fractions, using helical Tomotherapy®.
Results
Eighteen patients undergoing HALFMOON technique were compared to 54 subjects treated with conventionally contoured CTV plans. No difference was found in the planning target volume coverage between the two groups. Conversely, a statistically relevant dose reduction in HALFMOON patients was observed for ipsilateral lung (D15%, p < 0.0001; D20%, p < 0.0001; D35%, p = 0.003), contralateral lung (D20%, p = 0.048), contralateral breast (D15%, p = 0.031; D20%, p = 0.047), and stomach (Dmean, p = 0.011). Regarding the implant, V90% and D50% decreased by 46% and 8%, respectively, in the HALFMOON plans (p < 0.0001).
Conclusion
The HALFMOON approach is technically feasible and resulted in high-dose conformity of the target with a significant reduction of radiation dose delivered to implant and other organs. A clinical study is needed to assess the impact on reconstruction cosmetic outcome and local control.


Similar content being viewed by others
References
Aristei C, Kaidar-Person O, Tagliaferri L et al (2018) The assisi think tank meeting and survey of post MAstectomy Radiation Therapy after breast reconstruction: the ATTM-SMART report. Eur J Surg Oncol 44(4):436–443. https://doi.org/10.1016/j.ejso.2018.01.010
Ashenafi M, Boyd RA, Lee TK et al (2010) Feasibility of postmastectomy treatment with helical TomoTherapy. Int J Radiat Oncol Biol Phys 77(3):836–842. https://doi.org/10.1016/j.ijrobp.2009.06.02
Barry M, Kell MR (2011) Radiotherapy and breast reconstruction: a meta-analysis. Breast Cancer Res Treat 127:15–22. https://doi.org/10.1007/s10549-011-1401-x
Brennan ME, Flitcroft K, Warrier S, Snook K, Spillane AJ (2016) Immediate expander/implant breast reconstruction followed by post-mastectomy radiotherapy for breast cancer: aesthetic, surgical, satisfaction and quality of life outcomes in women with high-risk breast cancer. Breast 30:59–65. https://doi.org/10.1016/j.breast.2016.08.008
Buchholz TA, Strom EA, Perkins GH, McNeese MD (2002) Controversies regarding the use of radiation after mastectomy in breast cancer. Oncologist 7(6):539–546
Caudrelier JM, Meng J, Esche B, Grimard L, Ruddy T, Amjadi K (2014) IMRT sparing of normal tissues in loco-regional treatment of breast cancer. Radiat Oncol 9:161. https://doi.org/10.1186/1748-717X-9-161
Cavey ML, Bayouth JE, Endres EJ et al (2005) Dosimetric comparison of conventional and forward-planned intensity-modulated techniques for comprehensive locoregional irradiation of post-mastectomy left breast cancers. Med Dosim 30(2):107–116
Cordeiro PG, Albornoz CR, McCormick B, Hu Q, Van Zee K (2014) The impact of postmastectomy radiotherapy on two-stage implant breast reconstruction: an analysis of long-term surgical outcomes, aesthetic results and satisfaction over 13 years. Plast Reconstr Surg 134(4):588–595. https://doi.org/10.1097/PRS.0000000000000523
De Neve W, De Gersem W, Madani I (2012) Rational use of intensity-modulated radiation therapy: the importance of clinical outcome. Semin Radiat Oncol 22(1):40–49. https://doi.org/10.1016/j.semradonc.2011.09.003
EBCTCG (Early Breast Cancer Trialists’ Collaborative Group), McGale P, Taylor C, Correa C et al (2014) Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 383(9935):2127–2135. https://doi.org/10.1016/s0140-6736(14)60488-8
El-Sabawi B, Carey JN, Hagopian TM, Sbitany H, Patel KM (2016) Radiation and breast reconstruction: algorithmic approach and evidence-based outcomes. J Surg Oncol 133(8):906–912. https://doi.org/10.1002/jso.24143
Fowble B, Park C, Wang F et al (2015) Rates of reconstruction failure in patients undergoing immediate reconstruction with tissue expanders and/or implants and postmastectomy radiation therapy. Int J Radiat Oncol Biol Phys 92(3):634–641. https://doi.org/10.1016/j.ijrobp.2015.02.031
Frasier LL, Holden S, Holden T et al (2016) Temporal trends in postmastectomy radiation therapy and breast reconstruction associated with changes in National Comprehensive Cancer Network Guidellines. JAMA Oncol 2(1):95–101. https://doi.org/10.1001/jamaoncol.2015.3717
Gayzik FS, Yu MM, Danelson KA, Slice DE, Stitzel JD (2008) Quantification of age-related shape change of the human rib cage through geometric morphometrics. J Biomech 41(7):1545–1554. https://doi.org/10.1016/j.jbiomech.2008.02.006
ICRU Report (1993) Vol. 50. Prescribing, recording, and reporting photon beam therapy. Maryland: International Commission on Radiation Units and Measurements
Italian Association of Radiation Oncology (AIRO) (2013) Breast cancer Working Group. La Radioterapia Dei Tumori Della Mammella. Indicazioni e Criteri Guida. pp 75–81. http://www.radioterapiaitalia.it. Accessed 14 Jan 2019
Jagsi R, Moran J, Marsh R, Masi K, Griffith KA, Pierce LJ (2010) Evaluation of four techniques using intensity-modulated radiation therapy for comprehensive locoregional irradiation of breast cancer. Int J Radiat Oncol Biol Phys 78(5):1594–1603. https://doi.org/10.1016/j.ijrobp.2010.04.072
Kuske RR, Schuster R, Klein E, Young L, Perez CA, Fineberg B (1991) Radiotherapy and breast reconstruction: clinical results and dosimetry. Int J Radiat Oncol Biol Phys 21(2):339–346
Liljegren A, Unukovych D, Gagliardi G et al (2014) No difference in dose distribution in organs at risk in postmastectomy radiotherapy with or without breast implant reconstruction. Radiat Oncol 9:14. https://doi.org/10.1186/1748-717X-9-14
Mast M, Reynders T, Heijenbrok M et al (2016) Tangential IMRT versus TomoTherapy with and without breath-hold in left-sided whole breast irradiation. Acta Oncol 55(2):240–243. https://doi.org/10.3109/0284186X.2015.1046999
McGinley PH, Powell WR, Bostwick J (1980) Dosimetry of a silicone breast prosthesis. Radiology 135(1):223–224
Miles EA, Venables K, Hoskin PJ, Aird EG, START Trial Management Group (2009) Dosimetry and field matching for radiotherapy to the breast and supraclavicular fossa. Radiother Oncol. 91(1):42–48
Motwani SB, Strom EA, Schechter NR et al (2006) The impact of immediate breast reconstruction on the technical delivery of postmastectomy radiotherapy. Int J Radiat Oncol Biol Phys 66(1):76–82
Nissen HD, Yates ES, Andersen K et al (2018) PO-0918: consensus on target volume delineation and treatment planning strategy for DBCG RT Recontrial. Radiother Oncol 127(1):S492–S494. https://doi.org/10.1016/S0167-8140(18)31228-3
Offersen BV, Boersma LJ, Kirkove C et al (2015) ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol 114(1):3–10. https://doi.org/10.1016/j.radonc.2014.11.030
Ohri N, Cordeiro PG, Keam J et al (2012) Quantifying the impact of immediate reconstruction in postmastectomy radiation: a large, dose-volume histogram-based analysis. Int J Radiat Oncol Biol Phys 84(2):e153–e159. https://doi.org/10.1016/j.ijrobp.2012.03.026
Orecchia R, Rojas DP, Cattani F et al (2018) Hypofractionated postmastectomy radiotherapy with helical tomotherapy in patients with immediate breast reconstruction: dosimetric results and acute/intermediate toxicity evaluation. Med Oncol 35(3):39. https://doi.org/10.1007/s12032-018-1095-6
Osman SO, Hol S, Poortmans PM, Essers M (2014) Volumetric modulated arc therapy and breath-hold in image-guided locoregional left-sided breast irradiation. Radiother Oncol 112(1):17–22. https://doi.org/10.1016/j.radonc.2014.04.004
Recht A, Comen EA, Fine RE et al (2017) Postmastectomy radiotherapy: an American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Focused Guideline update. Ann Surg Oncol 24(1):38–51. https://doi.org/10.1245/s10434-016-5558-8
Ricotti R, Ciardo D, Fattori G et al (2017) Intra-fraction respiratory motion and baseline drift during breast Helical Tomotherapy. Radiother Oncol 122(1):79–86. https://doi.org/10.1016/j.radonc.2016.07.019
Sabino J, Lucas DJ, Shriver CD, Vertrees AE, Valerio IL, Singh DP (2016) NSQIP analysis: increased immediate reconstruction in the treatment of breast cancer. Am Surg 82(6):540–545
Schechter NR, Strom EA, Perkins GH et al (2005) Immediate breast reconstruction can impact postmastectomy irradiation. Am J Clin Oncol 28(5):485–494
Shedbalkar AR, Devata A, Padanilam T (1980) A study of effects of radiation on silicone prostheses. Plast Reconstr Surg 65(6):805–810
Vargo JA, Beriwal S (2015) RTOG chest wall contouring guidelines for post-mastectomy radiation therapy: is it evidence-based? Int J Radiat Oncol Biol Phys 93(2):266–267. https://doi.org/10.1016/j.ijrobp.2015.03.001
White J, Tai A, Arthur D, et al (2019) Breast cancer atlas for radiation therapy planning: consensus definitions. RTOG radiation therapy oncology group web site. http://www.rtog.org/CoreLab/ContouringAtlases/BreastCancerAtlas.aspx. Accessed 14 Jan 2019
Acknowledgements
The authors thank Piccolo Consilia, La Fauci Francesco and Gebbia Andrea for re-planning the control group in 3D-CRT. This work was partially supported by a research grant from Accuray Inc. entitled “Data collection and analysis of Tomotherapy and CyberKnife breast clinical studies, breast physics studies and prostate study” and by Fondazione IEO-CCM fellowship, project title “HALFMOON Study (Helical ALtered Fractionation for iMplant partial OmissiON): hypofractionated scheme of implant-sparing helical tomotherapy for intermediate/high-risk breast cancer”.
Funding
Miglietta E has received a research grant from Accuray Inc. (Data collection and analysis of Tomotherapy and CyberKnife breast clinical studies, breast physics studies, and prostate study). The sponsor did not play any role in the study design, collection, analysis, and interpretation of data, nor in the writing of the manuscript, nor in the decision to submit the manuscript for publication.
Spoto R has received a fellowship from Fondazione IEO-CCM for clinical implementation of HALFMOON study with the tile: “Helical ALtered Fractionation for iMplant partial OmissiON): Implant-sparing helical tomotherapy with hypofractionated scheme for intermediate/high-risk breast cancer”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Leonardi MC, Dell’Acqua V, Cattani F, and Jereczek-Fossa BA received a speaker honorarium from Accuracy International. All other authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the present study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
The dosimetric study has been approved by the institutional Ethical Committee as part of the research project entitled “Adjuvant radiation treatments with intensity-modulated radiotherapy and/or hypofractionated schedules for breast cancer” (26 May 2016, Milan, Italy).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Leonardi, M.C., Spoto, R., Miglietta, E. et al. HALFMOON TomoTherapy (Helical ALtered Fractionation for iMplant partial OmissiON): implant-sparing post-mastectomy radiotherapy reshaping the clinical target volume in the reconstructed breast. J Cancer Res Clin Oncol 145, 1887–1896 (2019). https://doi.org/10.1007/s00432-019-02938-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-019-02938-8