Abstract
Background
In the phase 3 BFORE trial (NCT02130557), treatment with bosutinib resulted in a significantly higher major molecular response rate at 12 months versus imatinib in the modified intent-to-treat (mITT) population of patients with newly diagnosed chronic phase chronic myeloid leukemia (CP CML). Assessment of patient-reported outcomes (PROs) was an exploratory objective.
Methods
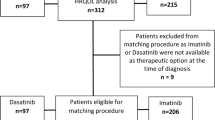
Patients with newly diagnosed CP CML were randomized 1:1 to receive once-daily bosutinib 400 mg or imatinib 400 mg as first-line therapy. Patients completed the Functional Assessment of Cancer Therapy-Leukemia (FACT-Leu) and EuroQoL-5 Dimensions (EQ-5D) questionnaires at baseline, every 3 months for the first 24 months of treatment, every 6 months thereafter, and at treatment completion. We report PRO results at month 12 in the mITT population (bosutinib: n = 246; imatinib: n = 241).
Results
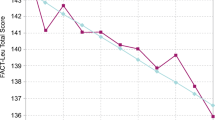
Mean FACT-Leu combined and subscale scores were similar at baseline in the bosutinib and imatinib arms; at month 12, all scores demonstrated improvement or maintenance of health-related quality of life (HRQoL) in both treatment arms. Repeated-measures mixed-effects models showed no significant difference between bosutinib and imatinib for any FACT-Leu score. Functional health status, as measured by EQ-5D, also demonstrated improvement or maintenance with bosutinib and imatinib at month 12.
Conclusions
Similar improvements in PROs compared with baseline were seen after 12 months of treatment with first-line bosutinib or imatinib in the BFORE trial. Newly diagnosed patients with CP CML receiving bosutinib or imatinib can preserve or improve HRQoL during treatment, although clinical efficacy was superior with bosutinib.



Similar content being viewed by others
Research data policy/data availability
Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
References
Anderson KR, Chambers CR, Lam N, Yau PS, Cusano F, Savoie ML, Sheikh N (2015) Medication adherence among adults prescribed imatinib, dasatinib, or nilotinib for the treatment of chronic myeloid leukemia. J Oncol Pharm Pract 21:19–25. https://doi.org/10.1177/1078155213520261
Bharmal M, Thomas J 3rd (2006) Comparing the EQ-5D and the SF-6D descriptive systems to assess their ceiling effects in the US general population. Value Health 9:262–271. https://doi.org/10.1111/j.1524-4733.2006.00108.x
Bower H, Bjorkholm M, Dickman PW, Hoglund M, Lambert PC, Andersson TM (2016) Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol 34:2851–2857. https://doi.org/10.1200/JCO.2015.66.2866
Brucker PS, Yost K, Cashy J, Webster K, Cella D (2005) General population and cancer patient norms for the functional assessment of cancer therapy-general (FACT-G). Eval Health Prof 28:192–211. https://doi.org/10.1177/0163278705275341
Cella D (1997) F.A.C.I.T. manual. Manual of the functional assessment of chronic illness therapy (FACIT) scales, v4. Center on Outcomes, Research and Education (CORE). Evanston Northwestern Healthcare, and Northwestern University, Evanston
Cella D et al (2012) Measuring health-related quality of life in leukemia: the functional assessment of cancer therapy-leukemia (FACT-Leu) questionnaire. Value Health 15:1051–1058. https://doi.org/10.1016/j.jval.2012.08.2210
Cortes J (2004) Natural history and staging of chronic myelogenous leukemia. Hematol Oncol Clin N Am 18:569–584, viii. https://doi.org/10.1016/j.hoc.2004.03.011
Cortes JE et al (2012) Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial. J Clin Oncol 30:3486–3492. https://doi.org/10.1200/JCO.2011.38.7522
Cortes JE et al (2018) Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol 36:231–237. https://doi.org/10.1200/JCO.2017.74.7162
De Marchi F, Medeot M, Fanin R, Tiribelli M (2017) How could patient reported outcomes improve patient management in chronic myeloid leukemia? Expert Rev Hematol 10:9–14. https://doi.org/10.1080/17474086.2017.1262758
Efficace F, Cannella L (2016) The value of quality of life assessment in chronic myeloid leukemia patients receiving tyrosine kinase inhibitors. Hematol Am Soc Hematol Educ Program 2016:170–179. https://doi.org/10.1182/asheducation-2016.1.170
Efficace F et al (2014a) International development of an EORTC questionnaire for assessing health-related quality of life in chronic myeloid leukemia patients: the EORTC QLQ-CML24. Qual Life Res 23:825–836. https://doi.org/10.1007/s11136-013-0523-5
Efficace F et al (2014b) Patient- versus physician-reporting of symptoms and health status in chronic myeloid leukemia. Haematologica 99:788–793. https://doi.org/10.3324/haematol.2013.093724
Efficace F et al (2018) Health-related quality of life in patients with chronic myeloid leukemia receiving first-line therapy with nilotinib. Cancer 124:2228–2237. https://doi.org/10.1002/cncr.31323
Eliasson L, Clifford S, Barber N, Marin D (2011) Exploring chronic myeloid leukemia patients’ reasons for not adhering to the oral anticancer drug imatinib as prescribed. Leuk Res 35:626–630. https://doi.org/10.1016/j.leukres.2010.10.017
EuroQol-Group (1990) EuroQol—a new facility for the measurement of health-related quality of life. Health Policy 16:199–208
Gambacorti-Passerini C et al (2011) Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J Natl Cancer Inst 103:553–561. https://doi.org/10.1093/jnci/djr060
Hahn EA et al (2003) Quality of life in patients with newly diagnosed chronic phase chronic myeloid leukemia on imatinib versus interferon alfa plus low-dose cytarabine: results from the IRIS study. J Clin Oncol 21:2138–2146. https://doi.org/10.1200/JCO.2003.12.154
Hehlmann R, Hochhaus A, Baccarani M, European L (2007) Chronic myeloid leukaemia. Lancet 370:342–350. https://doi.org/10.1016/S0140-6736(07)61165-9
Hughes TP et al (2010) Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International randomized study of interferon and STI571 (IRIS). Blood 116:3758–3765. https://doi.org/10.1182/blood-2010-03-273979
Ibrahim AR et al (2011) Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on long-term therapy. Blood 117:3733–3736. https://doi.org/10.1182/blood-2010-10-309807
Janssen B, Szende A (2014) Population norms for the EQ-5D. In: Szende A, Janssen B, Cabases J (eds) Self-reported population health: an international perspective based on EQ-5D. Springer, Dordrecht, pp 19–30. https://doi.org/10.1007/978-94-007-7596-1_3
Kantarjian HM, Deisseroth A, Kurzrock R, Estrov Z, Talpaz M (1993) Chronic myelogenous leukemia: a concise update. Blood 82:691–703
Kantarjian HM et al (2018) Long-term patient-reported outcomes from an open-label safety and efficacy study of bosutinib in Philadelphia chromosome-positive chronic myeloid leukemia patients resistant or intolerant to prior therapy. Cancer 124:587–595. https://doi.org/10.1002/cncr.31082
Lipton JH et al (2011) Health-related quality of life (HRQoL) in newly diagnosed patients (pts) with chronic phase chronic myelogenous leukemia (CP CML) treated with bosutinib (BOS) or imatinib (IM). J Clin Oncol 29:6612–6612. https://doi.org/10.1200/jco.2011.29.15_suppl.6612
Marin D et al (2010) Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol 28:2381–2388. https://doi.org/10.1200/JCO.2009.26.3087
Noens L et al (2009) Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood 113:5401–5411. https://doi.org/10.1182/blood-2008-12-196543
O’Brien SG et al (2003) Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 348:994–1004. https://doi.org/10.1056/NEJMoa022457
Pearman T, Yanez B, Peipert J, Wortman K, Beaumont J, Cella D (2014) Ambulatory cancer and US general population reference values and cutoff scores for the functional assessment of cancer therapy. Cancer 120:2902–2909. https://doi.org/10.1002/cncr.28758
Pfizer Inc (2017) Bosulif® (bosutinib) prescribing information. Pfizer Inc, New York
Pickard AS, Wilke CT, Lin HW, Lloyd A (2007) Health utilities using the EQ-5D in studies of cancer. Pharmacoeconomics 25:365–384. https://doi.org/10.2165/00019053-200725050-00002
Ruddy K, Mayer E, Partridge A (2009) Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin 59:56–66. https://doi.org/10.3322/caac.20004
Sasaki K et al (2015) Relative survival in patients with chronic-phase chronic myeloid leukaemia in the tyrosine-kinase inhibitor era: analysis of patient data from six prospective clinical trials. Lancet Haematol 2:e186–e193. https://doi.org/10.1016/S2352-3026(15)00048-4
Thompson PA, Kantarjian HM, Cortes JE (2015) Diagnosis and treatment of chronic myeloid leukemia in 2015. Mayo Clin Proc 90:1440–1454. https://doi.org/10.1016/j.mayocp.2015.08.010
Trask PC, Cella D, Besson N, Kelly V, Masszi T, Kim DW (2012) Health-related quality of life of bosutinib (SKI-606) in imatinib-resistant or imatinib-intolerant chronic phase chronic myeloid leukemia. Leuk Res 36:438–442. https://doi.org/10.1016/j.leukres.2011.10.011
van Reenan M, Oppe M (2015) EQ-5D-3L user guide, v5.1. Basic information on how to use the EQ-5D-3L instrument. EuroQol Research Foundation. https://euroqol.org/wp-content/uploads/2016/09/EQ-5D-3L_UserGuide_2015.pdf. Accessed Mar 28 2018
Whiteley J, Reisman A, Shapiro M, Cortes J, Cella D (2016) Health-related quality of life during bosutinib (SKI-606) therapy in patients with advanced chronic myeloid leukemia after imatinib failure. Curr Med Res Opin 32:1325–1334. https://doi.org/10.1185/03007995.2016.1174108
Williams LA et al (2013) Measuring the symptom burden associated with the treatment of chronic myeloid leukemia. Blood 122:641–647. https://doi.org/10.1182/blood-2013-01-477687
Zulbaran-Rojas A et al (2018) A prospective analysis of symptom burden for patients with chronic myeloid leukemia in chronic phase treated with frontline second- and third-generation tyrosine kinase inhibitors. Cancer Med 7:5457–5469. https://doi.org/10.1002/cam4.1808
Acknowledgements
This study (ClinicalTrials.gov, NCT02130557) was sponsored by Avillion LLP under a collaborative development agreement with Pfizer Inc. Medical writing support was provided by Joanna Bloom, PhD, of Engage Scientific Solutions and was funded by Pfizer. The authors would like to thank Irina Dyagil for her contributions to collection, assembly, and analysis of data, and Laurence Reilly and Allison Jeynes-Ellis of Avillion LLP, London, UK, for their contributions to study design and data analysis.
Funding
This study was sponsored by Avillion LLP under a collaborative development agreement with Pfizer Inc.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
JEC: consultancy (ARIAD, Bristol-Myers Squibb, ImmunoGen, Novartis, Pfizer, Takeda) and research funding (ARIAD, Bristol-Myers Squibb, ImmunoGen, Novartis, Sun Pharma, Pfizer, Takeda, Teva). CG-P: consultancy (Bristol-Myers Squibb) and honoraria and research funding (Pfizer). MWD: advisory board (Blue Print, Pfizer, Ascentage Pharma, and Humana), consultancy (Blue Print, Pfizer, Ascentage Pharma, and TRM), research funding (Pfizer), and study management committee (Blue Print and Takeda). MJM: consultancy (Bristol-Myers Squibb). CC: honoraria (Bristol-Myers Squibb, Chiltern, Novartis, and Otsuka) and travel (Pfizer). D-WK: consultancy (Bristol-Myers Squibb, Il-Yang, Novartis), honoraria, and research funding (Bristol-Myers Squibb, Il-Yang, Novartis, Pfizer), membership on board of directors or advisory committees (Bristol-Myers Squibb), and speakers bureau (Bristol-Myers Squibb, Novartis, Pfizer). DM: consultancy and honoraria (ARIAD, Bristol-Myers Squibb, Novartis, Pfizer) and honoraria and speakers bureau (Incyte). PlC: honoraria (ARIAD, Bristol-Myers Squibb, Incyte, Novartis, Pfizer) and research funding (Novartis). VG-G: consultancy, honoraria, and research funding (Bristol-Myers Squibb, Incyte, Novartis, Pfizer). RC: equity ownership (Pfizer and GlaxoSmithKline). CM: employment and equity ownership (Pfizer). AR: employment (Pfizer). AH: research funding (Bristol-Myers Squibb, Incyte, Novartis, Pfizer). THB: consultancy (Novartis, Pfizer, Janssen, Merck, Takeda) and research funding (Novartis, Pfizer).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cortes, J.E., Gambacorti-Passerini, C., Deininger, M.W. et al. Patient-reported outcomes in the phase 3 BFORE trial of bosutinib versus imatinib for newly diagnosed chronic phase chronic myeloid leukemia. J Cancer Res Clin Oncol 145, 1589–1599 (2019). https://doi.org/10.1007/s00432-019-02894-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-019-02894-3