Abstract
Purpose
The adequate second-line therapy of patients with glioblastoma (GBM) is a matter of ongoing debate. This particularly applies to patients with a non-methylated MGMT promotor who are known to have a poor response to alkylating chemotherapy. In some countries, antiangiogenic therapy with BEV is applied as second-line therapy, and in others nitrosourea therapy is second-line choice. It is an open question whether the delay of BEV to third-line therapy has a negative impact on survival.
Methods
A total of 61 adult patients (median age 56.9 years) with MGMT-non-methylated relapsed GBM treated with BEV (n = 45) or nitrosourea (n = 16) as second-line therapy were analyzed retrospectively and compared regarding progression-free survival (PFS) and overall survival (OS).
Results
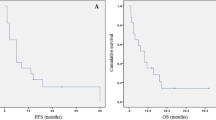
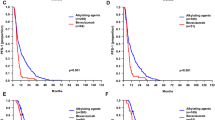
Patients treated with second-line BEV had longer median PFS (107 days, 95 % CI 80.7–133.2 days) than patients with second-line nitrosourea (52 days, 95 % CI 36.3–67.7 days, P = 0.011, logrank test). However, there was no significant difference in overall survival (BEV median 170 days, 95 % CI 87.2–252.8 days; nitrosourea median 256 days, 95 % CI 159.9–352.0 days, P = 0.468). PFS was similar after BEV third-line therapy (median 117 days, 95 % CI 23.6–210.4 days) as compared to second-line BEV therapy (median 107 days, 95 % CI 80.7–133.3 days, P = 0.584).
Conclusion
Our findings suggest that early treatment with BEV in patients with MGMT-non-methylated relapsed GBM is associated with a better PFS, but not with superior OS, possibly implicating that the early, i.e., second-line, use of BEV is not mandatory and BEV treatment may safely be delayed to third-line therapy in this subgroup of patients.



Similar content being viewed by others
References
Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, Brandes AA, Hilton M, Abrey L, Cloughesy T (2014) Bevacizumab plus radiotherapy–temozolomide for newly diagnosed glioblastoma. N Engl J Med 370:709–722. doi:10.1056/NEJMoa1308345
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740. doi:10.1200/JCO.2008.19.8721
Glas M, Hundsberger T, Stuplich M, Wiewrodt D, Kurzwelly D, Nguyen-Huu B, Rasch K, Herrlinger U (2009) Nimustine (ACNU) plus teniposide (VM26) in recurrent glioblastoma. Oncology 76:184–189. doi:10.1159/000201943
Hamza MA, Mandel JJ, Conrad CA, Gilbert MR, Yung WK, Puduvalli VK, DeGroot JF (2014) Survival outcome of early versus delayed bevacizumab treatment in patients with recurrent glioblastoma. J Neurooncol 119:135–140. doi:10.1007/s11060-014-1460-z
Happold C, Roth P, Wick W, Steinbach JP, Linnebank M, Weller M, Eisele G (2009) ACNU-based chemotherapy for recurrent glioma in the temozolomide era. J Neurooncol 92:45–48. doi:10.1007/s11060-008-9728-9
Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27:740–745. doi:10.1200/JCO.2008.16.3055
Mikeska T, Bock C, El-Maarri O, Hübner A, Ehrentraut D, Schramm J, Felsberg J, Kahl P, Büttner R, Pietsch T, Waha A (2007) Optimization of quantitative MGMT promoter methylation analysis using pyrosequencing and combined bisulfite restriction analysis. J Mol Diagn 9:368–381
Piccioni DE, Selfridge J, Mody RR, Chowdhury R, Li S, Lalezari S, Wawrzynski J, Quan J, Zurayk M, Chou AP, Sanchez DE, Liau LM, Ellingson BM, Pope WB, Nghiemphu PL, Green RM, Wang HJ, Yong WH, Elashoff R, Cloughesy TF, Lai A (2014) Deferred use of bevacizumab for recurrent glioblastoma is not associated with diminished efficacy. Neuro Oncol 16:815–822. doi:10.1093/neuonc/nou028
Schaub C, Tichy J, Schäfer N, Franz K, Mack F, Mittelbronn M, Kebir S, Thiepold AL, Waha A, Filmann N, Banat M, Fimmers R, Steinbach JP, Herrlinger U, Rieger J, Glas M, Bähr O (2016) Prognostic factors in recurrent glioblastoma patients treated with bevacizumab. J Neurooncol. doi:10.1007/s11060-016-2144-7
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466. doi:10.1016/S1470-2045(09)70025-7
Taal W, Oosterkamp HM, Walenkamp AM, Dubbink HJ, Beerepoot LV, Hanse MC, Buter J, Honkoop AH, Boerman D, de Vos FY, Dinjens WN, Enting RH, Taphoorn MJ, van den Berkmortel FW, Jansen RL, Brandsma D, Bromberg JE, van Heuvel I, Vernhout RM, van der Holt B, van den Bent MJ (2014) Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol 15:943–953. doi:10.1016/S1470-2045(14)70314-6
Tabouret E, Barrie M, Thiebaut A, Matta M, Boucard C, Autran D, Loundou A, Chinot O (2013) Limited impact of prognostic factors in patients with recurrent glioblastoma multiforme treated with a bevacizumab-based regimen. J Neurooncol 114:191–198. doi:10.1007/s11060-013-1170-y
Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Dowell JM, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Wagner M, Bigner DD, Friedman AH, Friedman HS (2007a) Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res 13:1253–1259
Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Marcello J, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Sampson J, Wagner M, Bailey L, Bigner DD, Friedman AH, Friedman HS (2007b) Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 25:4722–4729
Weller M, van den Bent M, Hopkins K, Tonn JC, Stupp R, Falini A, Cohen-Jonathan-Moyal E, Frappaz D, Henrikson R, Balana C, Chinot O, Ram Z, Reifenberger G, Soffietti R, Wick W (2014) EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol 15:e395–e403. doi:10.1016/S1470-2045(14)70011-7
Weller M, Tabatabai G, Kästner B, Felsberg J, Steinbach JP, Wick A, Schnell O, Hau P, Herrlinger U, Sabel MC, Wirsching HG, Ketter R, Bähr O, Platten M, Tonn JC, Schlegel U, Marosi C, Goldbrunner R, Stupp R, Homicsko K, Pichler J, Nikkhah G, Meixensberger J, Vajkoczy P, Kollias S, Hüsing J, Reifenberger G, Wick W, DIRECTOR Study Group (2015) MGMT promoter methylation is a strong prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: the director trial. Clin Cancer Res 21:2057–2064. doi:10.1158/1078-0432.CCR-14-2737
Wick W, Puduvalli VK, Chamberlain MC, van den Bent MJ, Carpentier AF, Cher LM, Mason W, Weller M, Hong S, Musib L, Liepa AM, Thornton DE, Fine HA (2010) Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol 28:1168–1174. doi:10.1200/JCO.2009.23.2595
Wick W, Brandes AA, Gorlia T, Bendszus M, Sahm F, Taal W, Taphoorn M, Domont J, Idbaih A, Campone M, Clement PM, Stupp R, Fabbro M, Dubois F, Bais C, Musmeci D, Platten M, Weller M, Golfinopoulos, van den Bent M (2015) Phase III trial exploring the combination of bevacizumab and lomustine in patients with first recurrence of a glioblastoma: the EORTC 26101 trial. In: Neuro-Oncology, 20th Annual Scientific Meeting of the Society for Neuro-Oncology, 2015
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ulrich Herrlinger has served as a consultant for Roche, Novocure, Mundipharma and Bristol-Myers-Squibb. He has received a scientific Grant from Roche and speakers honoraria from Roche, Medac and Riemser Pharma. Martin Glas has served as a consultant for Roche, Novartis, Mundipharma, sigma tau and UCB. He has received speakers honoraria from Roche, Medac and sigma tau, and a scientific Grant from Medac. Niklas Schäfer received honoraria from Roche. All other authors declare that they have no conflict of interest.
Ethical approval and informed consent
All procedures performed in our study were in accordance with the ethical standards of the institutional research committee (local Ethic Committee) and with the 1964 Helsinki declaration and its later amendments. For this type of study, formal consent is not required.
Additional information
Ulrich Herrlinger and Martin Glas have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schaub, C., Schäfer, N., Mack, F. et al. The earlier the better? Bevacizumab in the treatment of recurrent MGMT-non-methylated glioblastoma. J Cancer Res Clin Oncol 142, 1825–1829 (2016). https://doi.org/10.1007/s00432-016-2187-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-016-2187-3