Abstract
Purpose
Predicting the feasibility of platinum-based chemotherapy remains an important issue in elderly (over 70 years) patients with non-small cell lung cancer (NSCLC). The aim of this study was to identify the risk factors for the early serious adverse events (SAEs) (during cycles 1–2) in elderly receiving platinum-based chemotherapy, and to explore the clinical characteristics of patients who require early treatment termination without progressive disease (PD).
Methods
One hundred and ninety-eight consecutive elderly NSCLC patients receiving platinum-based chemotherapy were retrospectively reviewed.
Results
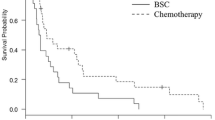
The median age was 73 years (range 70–83). 161 (81 %) were males, and 190 (95 %) were PS 0–1. Fifty-one (29 %) and 39 (19 %) patients developed early non-hematological SAEs and hematological SAEs, respectively. Multivariate analysis identified low serum albumin (<3.0 g/dl) as an independent risk factor for non-hematological SAEs, while low creatinine clearance (<45 ml/min) for hematological SAEs. In all, 24 (12 %) patients needed early treatment termination without PD. The major reason for this event was the development of non-hematological SAEs (4.5 %), followed by grade 2 non-hematological adverse events (AEs) (3 %). In multivariate analysis, age over 75 years and low serum albumin were associated with this event. The median overall survival (OS) in patients with this event was only 6.0 months, while the development of early SAE was not associated with poor OS.
Conclusion
Baseline serum albumin might be useful for predicting the feasibility of platinum-based chemotherapy, and the risk estimation of early treatment termination without PD might be beneficial for the treatment selection in elderly NSCLC patients.



Similar content being viewed by others
References
Abe T et al (2015) Randomized phase III trial comparing weekly docetaxel plus cisplatin versus docetaxel monotherapy every 3 weeks in elderly patients with advanced non-small-cell lung cancer the intergroup trial JCOG0803/WJOG4307L. J Clin Oncol. doi:10.1200/JCO.2014.55.8627
Akbari A et al (2014) Canadian society of nephrology commentary on the KDIGO clinical practice guideline for CKD evaluation and management. Am J Kidney Dis. doi:10.1053/j.ajkd.2014.10.013
Arrieta O et al (2010) Association of nutritional status and serum albumin levels with development of toxicity in patients with advanced non-small cell lung cancer treated with paclitaxel-cisplatin chemotherapy: a prospective study. BMC Cancer 10:50. doi:10.1186/1471-2407-10-50
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Colinet B, Jacot W, Bertrand D, Lacombe S, Bozonnat MC, Daures JP, Pujol JL (2005) A new simplified comorbidity score as a prognostic factor in non-small-cell lung cancer patients: description and comparison with the Charlson’s index. Br J Cancer 93:1098–1105. doi:10.1038/sj.bjc.6602836
De Santis M et al (2009) Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer “unfit” for cisplatin-based chemotherapy: phase II–results of EORTC study 30986. J Clin Oncol 27:5634–5639. doi:10.1200/JCO.2008.21.4924
De Santis M et al (2012) Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol 30:191–199. doi:10.1200/JCO.2011.37.3571
Extermann M et al (2012) Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 118:3377–3386. doi:10.1002/cncr.26646
Fearon K et al (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12:489–495. doi:10.1016/S1470-2045(10)70218-7
Fukuse T, Satoda N, Hijiya K, Fujinaga T (2005) Importance of a comprehensive geriatric assessment in prediction of complications following thoracic surgery in elderly patients. Chest 127:886–891. doi:10.1378/chest.127.3.886
Gajra A, Jatoi A (2014) Non-small-cell lung cancer in elderly patients: a discussion of treatment options. J Clin Oncol. doi:10.1200/JCO.2014.55.3099
Gioulbasanis I, Baracos VE, Giannousi Z, Xyrafas A, Martin L, Georgoulias V, Mavroudis D (2011) Baseline nutritional evaluation in metastatic lung cancer patients: Mini Nutritional Assessment versus weight loss history. Ann Oncol Off J Eur Soc Med Oncol/ESMO 22:835–841. doi:10.1093/annonc/mdq440
Gridelli C et al (2003) Chemotherapy for elderly patients with advanced non-small-cell lung cancer: the Multicenter Italian Lung Cancer in the Elderly Study (MILES) phase III randomized trial. J Natl Cancer Inst 95:362–372
Hurria A et al (2011) Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 29:3457–3465. doi:10.1200/JCO.2011.34.7625
Jacot W, Colinet B, Bertrand D, Lacombe S, Bozonnat MC, Daures JP, Pujol JL (2008) Quality of life and comorbidity score as prognostic determinants in non-small-cell lung cancer patients. Ann Oncol 19:1458–1464. doi:10.1093/annonc/mdn064
Kaneko S et al (2003) Projection of lung cancer mortality in Japan. Cancer Sci 94:919–923
Kudoh S et al (2006) Phase III study of docetaxel compared with vinorelbine in elderly patients with advanced non-small-cell lung cancer: results of the West Japan Thoracic Oncology Group Trial (WJTOG 9904). J Clin Oncol 24:3657–3663. doi:10.1200/JCO.2006.06.1044
Min LC, Elliott MN, Wenger NS, Saliba D (2006) Higher vulnerable elders survey scores predict death and functional decline in vulnerable older people. J Am Geriatr Soc 54:507–511. doi:10.1111/j.1532-5415.2005.00615.x
Ohe Y et al (2007) Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer Four-Arm Cooperative Study in Japan. Ann Oncol 18:317–323. doi:10.1093/annonc/mdl377
Park JO et al (2007) Phase III trial of two versus four additional cycles in patients who are nonprogressive after two cycles of platinum-based chemotherapy in non small-cell lung cancer. J Clin Oncol 25:5233–5239. doi:10.1200/jco.2007.10.8134
Quoix E et al (2011) Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet 378:1079–1088. doi:10.1016/S0140-6736(11)60780-0
Rossi A et al (2014) Six versus fewer planned cycles of first-line platinum-based chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol 15:1254–1262. doi:10.1016/S1470-2045(14)70402-4
Schiller JH et al (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. New Engl J Med 346:92–98. doi:10.1056/NEJMoa011954
Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics, 2014. CA Cancer J Clin 64:9–29. doi:10.3322/caac.21208
The Elderly Lung Cancer Vinorelbine Italian Study Group (1999) Effects of vinorelbine on quality of life and survival of elderly patients with advanced non-small-cell lung cancer. J Natl Cancer Inst 91:66–72
Tsukada H et al (2015) Randomized controlled trial comparing docetaxel-cisplatin combination with weekly docetaxel alone in elderly patients with advanced non-small-cell lung cancer: Japan Clinical Oncology Group (JCOG) 0207dagger. Jpn J Clin Oncol 45:88–95. doi:10.1093/jjco/hyu176
Vincent MD, Dranitsaris G (2009) The price function of toxicity. Lancet Oncol 10:299–303. doi:10.1016/S1470-2045(09)70067-1
von Plessen C et al (2006) Palliative chemotherapy beyond three courses conveys no survival or consistent quality-of-life benefits in advanced non-small-cell lung cancer. Br J Cancer 95:966–973. doi:10.1038/sj.bjc.6603383
Acknowledgments
This work was supported by Central Japan Lung Study Group.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have received no funding support for this work. Information about financial relationships outside the work is as follows: Dr. Morise has received speaking honoraria from Chugai Pharmaceutical Co., TAIHO Pharmaceutical Co., Eli Lilly Japan, and Pfizer Inc.; Dr. Ando has received speaking honoraria from Merck Serono Co.; Dr. Ogasawara has received speaking honoraria from Pfizer Inc., KYORIN Pharmaceutical Co., Glaxo Smith Kline K.K., Novartis Pharma Co., Meiji Seika Pharma Co., and Boehringer Ingerheim; Dr. Shindo has received speaking honoraria from Daiichi Sankyo, KYORIN Pharmaceutical Co., TAIHO PHARMACEUTICAL CO., MSD K.K., Kyowa Hakko Kirin co., Glaxo Smith Kline K.K., and Nippon Boehringer Ingelheim Co; Dr. Matsumoto has received speaking honoraria from TEIJIN LIMITED., Philips Respironics GK., Chugai Pharmaceutical Co., and DAIICHI SANKYO Co; Dr. Sugino has received speaking honoraria from Astellas Pharma Inc., Ono Pharmaceutical Co., Shionogi & Co., Abbott Japan Co., Ltd., MSD K.K., Sanofi, GlaxoSmithKline plc., Mitsubishi Tanabe Pharma., Pfizer Inc., Novartis Pharma K.K., AstraZeneca, and KYORIN Pharmaceutical Co.; Dr. Hase has received speaking honoraria from Chugai Pharmaceutical Co., AstraZeneca, Boehringer Ingerheim, Novartis Pharma, DAIICHI SANKYO COMPANY; Dr. Kondo has received speaking honoraria from Chugai Pharmaceutical Co., AstraZeneca, Boehringer Ingerheim, Novartis Pharma K.K., TAIHO Pharmaceutical Co., Pfizer Inc., and Eli Lily Japan K.K.; Dr. Saito reported a grant from ONO PHARMACEUTICAL CO., Merck Serno, TAIHO Pharmaceutical Co., Daiichi Sankyo, Daiichi Sankyo; Dr. Hasegawa reported receiving grant from Boehringer Ingelheim., Pfizer Inc., Astellas Pharma Inc., ONO PHARMACEUTICAL CO., Shionogi & Co., AstraZeneca, Sanofi K.K., TEIJIN LIMITED, MSD K.K., Meiji Seika Pharma Co., DAIICHI SANKYO COMPANY, LIMITED., GlaxoSmithKline K.K., Otsuka Pharmaceutical Co., KYORIN Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Novartis Pharma K. K., Kyowa Hakko Kirin Co., Eli Lilly Japan K.K., Chugai Pharmaceutical Co. that was paid to Nagoya University.
Ethical standards
All procedures performed in studies were in accordance with the ethical standards of the Institutional Research Board and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
This study design was the retrospective observational cohort study with no invasiveness for patients. All data were obtained by reviewing medical charts. Therefore, informed consent for each patient was not required according to the Japanese guideline of cohort study. This study was approved by Ethics Committee of all institutions.
Additional information
Toshio Kato and Masahiro Morise have contributed equally to this article
Rights and permissions
About this article
Cite this article
Kato, T., Morise, M., Ando, M. et al. Can we predict the development of serious adverse events (SAEs) and early treatment termination in elderly non-small cell lung cancer (NSCLC) patients receiving platinum-based chemotherapy?. J Cancer Res Clin Oncol 142, 1629–1640 (2016). https://doi.org/10.1007/s00432-016-2170-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-016-2170-z