Abstract
Purpose
Malignant hematological diseases represent the most common pediatric cancer. As they cannot always be cured by chemotherapy alone, leukemia and myelodysplastic syndrome (MDS) are frequent medical indications for hematopoietic stem cell transplantation, yet even this treatment is not capable of preventing relapse for certain. Therefore, molecular markers are used to monitor minimal residual disease (MRD) to be enabled to react early to an impeding relapse. As specific markers are not always available, Wilms’ tumor gene 1 (WT1) has been suggested as a universal marker, but has not yet been established clinically.
Methods
We determined the level of WT1 gene expression in 130 children, adolescents and young adults with malignant hematological diseases prior to transplantation and evaluated its impact on patients’ outcome. A real-time quantitative RT-PCR was used for this purpose.
Results
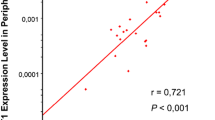
The relationship between a high level of WT1 and the cumulative incidence of relapse, event-free survival and overall survival proved to be highly significant in univariate and multivariate analyses. Forty-eight percent of all patients with high WT1 levels suffered from a relapse, whereas only eight percent showing normal WT1 levels before transplantation relapsed. The most convincing result was found for acute myeloid leukemia (AML) and MDS.
Conclusion
We conclude that WT1 expression prior to transplantation qualifies as an independent prognostic factor and should be further evaluated for MRD monitoring. It might especially be useful for patients with AML or MDS missing specific markers.







Similar content being viewed by others
References
Bader P et al (2004) WT1 gene expression: useful marker for minimal residual disease in childhood myelodysplastic syndromes and juvenile myelo-monocytic leukemia? Eur J Haematol 73:25–28. doi:10.1111/j.1600-0609.2004.00260.x
Boublikova L et al (2006) Wilms’ tumor gene 1 (WT1) expression in childhood acute lymphoblastic leukemia: a wide range of WT1 expression levels, its impact on prognosis and minimal residual disease monitoring. Leukemia 20:254–263. doi:10.1038/sj.leu.2404047
Call KM et al (1990) Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms’ tumor locus. Cell 60:509–520
Cazzaniga G, dA E, Corral L, Biondi A (2003) Results of minimal residual disease (MRD) evaluation and MRD-based treatment stratification in childhood ALL. Best pract res Clin haematol 15:623–638
Cilloni D, Saglio G (2004) WT1 as a universal marker for minimal residual disease detection and quantification in myeloid leukemias and in myelodysplastic syndrome. Acta Haematol 112:79–84. doi:10.1159/000077562
Cilloni D et al (2003) Significant correlation between the degree of WT1 expression and the International Prognostic Scoring System Score in patients with myelodysplastic syndromes. J clin oncol: Off J American Soc Clin Oncol 21:1988–1995. doi:10.1200/JCO.2003.10.503
Gaiger A et al (1999) Wilms’ tumour gene (wt1) expression at diagnosis has no prognostic relevance in childhood acute lymphoblastic leukaemia treated by an intensive chemotherapy protocol. Eur J Haematol 63:86–93
Hosen N et al (2002) Very low frequencies of human normal CD34 + haematopoietic progenitor cells express the Wilms’ tumour gene WT1 at levels similar to those in leukaemia cells. Br J Haematol 116:409–420
Inoue K, Sugiyama H, Ogawa H, Nakagawa M, Yamagami T, Miwa H et al (1994) WT1 as a new prognostic factor and a new marker for the detection of minimal residual disease in acute leukemia. Blood 84(9):3071–3079
Jacobsohn DA et al (2009) High WT1 gene expression before haematopoietic stem cell transplant in children with acute myeloid leukaemia predicts poor event-free survival. Br J Haematol 146:669–674. doi:10.1111/j.1365-2141.2009.07770.x
Kwon M et al (2012) Evaluation of minimal residual disease by real-time quantitative PCR of Wilms’ tumor 1 expression in patients with acute myelogenous leukemia after allogeneic stem cell transplantation: correlation with flow cytometry and chimerism. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplantation 18:1235–1242. doi:10.1016/j.bbmt.2012.01.012
Lange T et al (2011) Monitoring of WT1 expression in PB and CD34(+) donor chimerism of BM predicts early relapse in AML and MDS patients after hematopoietic cell transplantation with reduced-intensity conditioning. Leukemia 25:498–505. doi:10.1038/leu.2010.283
Ogawa H et al (2003) The usefulness of monitoring WT1 gene transcripts for the prediction and management of relapse following allogeneic stem cell transplantation in acute type leukemia. Blood 101:1698–1704. doi:10.1182/blood-2002-06-1831
Rossi G et al (2012) Comparison between multiparameter flow cytometry and WT1-RNA quantification in monitoring minimal residual disease in acute myeloid leukemia without specific molecular targets. Leuk Res 36:401–406. doi:10.1016/j.leukres.2011.11.020
Sugiyama H (2001) Wilms’ tumor gene WT1: its oncogenic function and clinical application. Int J Hematol 73:177–187
Sugiyama H (2010) WT1 (Wilms’ tumor gene 1): biology and cancer immunotherapy. Jpn J Clin Oncol 40:377–387. doi:10.1093/jjco/hyp194
Tamaki H et al (1999) The Wilms’ tumor gene WT1 is a good marker for diagnosis of disease progression of myelodysplastic syndromes. Leukemia 13:393–399
Trka J et al (2002) Real-time quantitative PCR detection of WT1 gene expression in children with AML: prognostic significance, correlation with disease status and residual disease detection by flow cytometry. Leukemia 16:1381–1389. doi:10.1038/sj.leu.2402512
Valkova V et al (2013) Minimal residual disease detectable by quantitative assessment of WT1 gene before allogeneic stem cell transplantation in patients in first remission of acute myeloid leukemia has an impact on their future prognosis. Clin Transplant 27:E21–29. doi:10.1111/ctr.12046
Weisser M, Kern W, Rauhut S, Schoch C, Hiddemann W, Haferlach T, Schnittger S (2005) Prognostic impact of RT-PCR-based quantification of WT1 gene expression during MRD monitoring of acute myeloid leukemia. Leukemia 19:1416–1423. doi:10.1038/sj.leu.2403809
Zhao XS et al (2013) Combined use of WT1 and flow cytometry monitoring can promote sensitivity of predicting relapse after allogeneic HSCT without affecting specificity. Ann Hematol 92:1111–1119. doi:10.1007/s00277-013-1733-1
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Woehlecke, C., Wittig, S., Arndt, C. et al. Prognostic impact of WT1 expression prior to hematopoietic stem cell transplantation in children with malignant hematological diseases. J Cancer Res Clin Oncol 141, 523–529 (2015). https://doi.org/10.1007/s00432-014-1832-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-014-1832-y