Abstract
Purpose
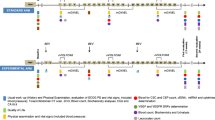
Significant prolongation of overall survival (OS) has been reached in metastatic colorectal cancer (mCRC) treatment within the last 5–10 years. Our study was conducted in order to evaluate and compare OS of different standard of care treatment options in a university-based outpatient clinic.
Methods
One hundred and three mCRC patients were identified by retrospective analysis and treated according to available guidelines. OS was analyzed according to the different combinations of first- and second-line treatments.
Results
mCRC patients revealed an mOS of 34.4 months. Patients receiving anti-vascular endothelial growth factor (VEGF) blockade in at least one treatment line showed a significantly longer survival time (p = 0.0056) versus patients without any bevacizumab. No OS differences were detected comparing the different first- and second-line chemotherapy (CTX) strategies in the unselected population. However, wild-type (wt) Kras patients treated with anti-epidermal growth factor receptor (EGFR) therapy plus CTX in first-line therapy showed significantly longer OS compared to those receiving only additional VEGF inhibition or no targeted therapy (p = 0.0056; mOS 46.8 vs. 20.4 months, respectively). wt Kras patients profited in trend (p = 0.076) from CTX combinations of first-line anti-EGFR followed by second-line anti-VEGF compared to first-line anti-VEGF followed by second-line anti-EGFR (mOS 46.8 vs. 19.2 months, respectively).
Conclusions
Our results indicate successful allocation of the current mCRC treatment according to the Kras status. Differences in OS of wt Kras patients indicated the further need for randomized trials to define the potential benefit of sequential therapy with EGFR inhibition in first-line therapy followed by VEGFR inhibition vice versa.



Similar content being viewed by others
Abbreviations
- OS:
-
Overall survival
- mCRC:
-
Metastatic colorectal cancer
- CTX:
-
Chemotherapy
- VEGF:
-
Vascular endothelial growth factor
- EGFR:
-
Epidermal growth factor receptor
- TK:
-
Tyrosine kinases
- TTFS:
-
Time to failure of strategy
- DCR:
-
Disease control rate
- CR:
-
Complete response
- PR:
-
Partial response
- SD:
-
Stable disease
- USE:
-
Undesirable side effects
- PD:
-
Progressive disease
- wt:
-
Wild type
- EOT:
-
End of treatment
References
Awada A, Gil T, Whenham N, Van Hamme J, Besse-Hammer T, Brendel E et al (2011) Safety and pharmacokinetics of sorafenib combined with capecitabine in patients with advanced solid tumors: results of a phase 1 trial. J Clin Pharmacol 51:1674–1684. doi:10.1177/0091270010386226
Bennouna J, Sastre J, Arnold D, Osterlund P, Greil R, Van Cutsem E et al (2013) Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 14:29–37. doi:10.1016/S1470-2045(12)70477-1
Blesa JM, Pulido EG (2010) Colorectal cancer: response to sunitinib in a heavily pretreated colorectal cancer patient. Anticancer Drugs 21(Suppl 1):S23–S26. doi:10.1097/01.cad.0000361533.36428.0b
Chibaudel B, Tournigand C, Andre T, de Gramont A (2012) Therapeutic strategy in unresectable metastatic colorectal cancer. Ther Adv Med Oncol 4:75–89. doi:10.1177/1758834011431592
Cutsem EV, Nowacki M, Lang I, Cascinu S, Shchepotin I, Maurel J et al (2007) Randomized phase III study of irinotecan and 5-FU/FA with or without cetuximab in the first-line treatment of patients with metastatic colorectal cancer (mCRC): the CRYSTAL trial. J Clin Oncol 25:4000
Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M et al (2010) Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 28:4697–4705. doi:10.1200/JCO.2009.27.4860
Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C et al (2007) Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol 25:1670–1676. doi:10.1200/JCO.2006.09.0928
Fisher GA, Kuo T, Ramsey M, Schwartz E, Rouse RV, Cho CD et al (2008) A phase II study of gefitinib, 5-fluorouracil, leucovorin, and oxaliplatin in previously untreated patients with metastatic colorectal cancer. Clin Cancer Res 14:7074–7079. doi:10.1158/1078-0432.CCR-08-1014
Heinemann V, Weikersthal LFV, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran S-E et al. (2013) Randomized comparison of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment of KRAS wild-type metastatic colorectal cancer: German AIO study KRK-0306 (FIRE-3). J Clin Oncol 31: LBA3506
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W et al (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342. doi:10.1056/NEJMoa032691350/23/2335
Hurwitz HI, Fehrenbacher L, Hainsworth JD, Heim W, Berlin J, Holmgren E et al (2005) Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol 23:3502–3508. doi:10.1200/JCO.2005.10.017
International Agency for Research on Cancer (2010) GLOBOCAN 2008 v2.0, Estimated cancer incidence, mortality prevalence and disability-adjusted life years (DALYs) worldwide in 2008. http://globocan.iarc.fr/
Kelley RK, Hwang J, Magbanua MJ, Watt L, Beumer JH, Christner SM et al (2013) A phase 1 trial of imatinib, bevacizumab, and metronomic cyclophosphamide in advanced colorectal cancer. Br J Cancer 109:1725–1734. doi:10.1038/bjc.2013.553
Kindler HL, Friberg G, Skoog L, Wade-Oliver K, Vokes EE (2005) Phase I/II trial of gefitinib and oxaliplatin in patients with advanced colorectal cancer. Am J Clin Oncol 28:340–344
Lawrence YR, Golan T, Aderka D, Shani A, Zach L, Ben-David M et al (2012) Changing prognosis of metastatic colorectal adenocarcinoma (mCRC) 1988–2008 within the general population: has everyone benefitted? J Clin Oncol 30:e14143
Lenz HJ, Van Cutsem E, Khambata-Ford S, Mayer RJ, Gold P, Stella P et al (2006) Multicenter phase II and translational study of cetuximab in metastatic colorectal carcinoma refractory to irinotecan, oxaliplatin, and fluoropyrimidines. J Clin Oncol 24:4914–4921. doi:10.1200/JCO.2006.06.7595
Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Trenta P et al (2013) FOLFOXIRI plus bevacizumab (bev) versus FOLFIRI plus bev as first-line treatment of metastatic colorectal cancer (MCRC): results of the phase III randomized TRIBE trial. J Clin Oncol 31:336
Moehler M, Mueller A, Trarbach T, Lordick F, Seufferlein T, Kubicka S et al (2010) Cetuximab with irinotecan, folinic acid and 5-fluorouracil as first-line treatment in advanced gastroesophageal cancer: a prospective multi-center biomarker-oriented phase II study. Ann Oncol 22:1358–1366. doi:10.1093/annonc/mdq591
Oliner KS, Douillard J-Y, Siena S, Tabernero J, Burkes RL, Barugel ME et al. (2013) Analysis of KRAS/NRAS and BRAF mutations in the phase III PRIME study of panitumumab (pmab) plus FOLFOX versus FOLFOX as first-line treatment (tx) for metastatic colorectal cancer (mCRC). J Clin Oncol 31. Abstract 3511
Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y et al (2010) Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 28:4706–4713. doi:10.1200/JCO.2009.27.6055
Qvortrup C, Jensen BV, Jorgensen TL, Nielsen D, Bjerregaard JK, Pfeiffer P (2010) Addition of sunitinib to cetuximab and irinotecan in patients with heavily pre-treated advanced colorectal cancer. Acta Oncol 49:833–836. doi:10.3109/0284186X.2010.482104
Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R et al (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26:2013–2019. doi:10.1200/JCO.2007.14.9930
Samalin E, Bouche O, Thezenas S, Francois E, Adenis A, Bennouna J et al (2014) Sorafenib and irinotecan (NEXIRI) as second- or later-line treatment for patients with metastatic colorectal cancer and KRAS-mutated tumours: a multicentre Phase I/II trial. Br J Cancer 110:1148–1154. doi:10.1038/bjc.2013.813
Shike M, Winawer SJ, Greenwald PH, Bloch A, Hill MJ, Swaroop SV (1990) Primary prevention of colorectal cancer. The WHO collaborating centre for the prevention of colorectal cancer. Bull World Health Organ 68:377–385
Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP et al (2008) EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 26:2311–2319. doi:10.1200/JCO.2007.13.1193
Stintzing S, Jung A, Rossius L, Modest DP, Fischer von Weikersthal L, Decker T et al. (2013) Analysis of KRAS/NRAS and BRAF mutations in FIRE-3: A randomized phase III study of FOLFIRI plus cetuximab or bevacizumab as first-line treatment for wild-type (WT) KRAS (exon 2) metastatic colorectal cancer (mCRC) patients. European Cancer Congress 2013. Abstract 17
Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D et al (2004) FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 22:229–237. doi:10.1200/JCO.2004.05.113
Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B et al (2007) Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 25:1658–1664. doi:10.1200/JCO.2006.08.1620
Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A et al (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360:1408–1417. doi:10.1056/NEJMoa0805019
Acknowledgments
The authors are grateful to all investigators and patients who participated in the trial. We thank Arno Schad for technical assistance and the excellent collaboration. The manuscript was reviewed by all authors.
Conflict of interest
M Moehler received honoraria for presentations in satellite symposia by Merck and Roche. The rest of the authors have no competing interests to declare. Medical writing by GSOmbH (ALK and JMW) was supported by an unrestricted grant of Merck Serono. However, ALK and JMW did not prepare the first draft and did not contribute to critical revisions, interpretation of results, or any critical intellectual impact.
Author information
Authors and Affiliations
Corresponding author
Additional information
Markus Moehler and Thomas Thomaidis have contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Moehler, M., Thomaidis, T., Zeifri, C. et al. Inclusion of targeted therapies in the standard of care for metastatic colorectal cancer patients in a German cancer center: the more the better?!. J Cancer Res Clin Oncol 141, 515–522 (2015). https://doi.org/10.1007/s00432-014-1829-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-014-1829-6