Abstract
Purposes
The overall prognosis for recurrent malignant glioma (MG) is extremely poor, and treatment options are limited. We evaluated our multicenter retrospective experience for patients with recurrent MG administering bevacizumab and irinotecan in combination therapy.
Methods
A total of 115 patients with grade IV glial tumor (n = 93) and grade III glial tumor (n = 22) were retrospectively evaluated at 14 centers in Turkey. Primary objectives of the study were to evaluate the efficacy and toxicity of the bevacizumab and irinotecan as salvage treatment based on response to therapy, progression-free survival (PFS), 6 months of PFS, overall survival (OS), and 6 months of OS (OS6).
Results
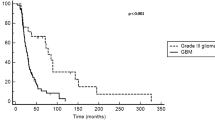
Bevacizumab and irinotecan were performed as second line (79.1 %) and third line treatment (20.9 %). Median chemotherapy cycle was 6 (range 1–37), and median follow-up was 6 months (range 1–36 months). Objective response rate was 39.1 %. Six-month PFS and OS6 were 46.3 % and 67.5 %, respectively. Median PFS was 6 months (95 % CI 2.5–9.5) and 6 months (95 % CI 4.9–7.1) in the grade III and IV groups, respectively (p = 0.773). Median OS was 9 months (95 % CI 7.1–10.9) and 8 months (95 % CI 6.6–9.4) in the grade III and IV groups, respectively (p = 0.450). Serious toxicities were observed in 7.8 % of patients. Treatment-related toxic death was observed in 3 patients. There was no treatment related to central nervous system hemorrhage or other serious hemorrhages.
Conclusions
Present study results were consistent with previous studies. In addition, we detected similar outcomes in grade III and IV glial tumors.


Similar content being viewed by others
References
Bokstein F, Shpigel S, Blumenthal DT (2008) Treatment with bevacizumab and irinotecan for recurrent high-grade glial tumors. Cancer 112:2267–2273
Brandes AA, Tosoni A, Amistà P, Nicolardi L, Grosso D, Berti F et al (2004) How effective is BCNU in recurrent glioblastoma in the modern era? A phase II trial. Neurology 63:1281–1284
Buckner JC (2003) Factors influencing survival in high-grade gliomas. Semin Oncol 30:10–14
Cancer Therapy Evaluation Program. Common Terminology Criteria For Adverse Events, version 3, 9 Aug (2006). http://ctepcancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed 15 Oct, 2012
Demirci U, Yaman M, Buyukberber S, Coskun U, Baykara M, Uslu K et al (2012) Prognostic importance of markers for inflammation, angiogenesis and apoptosis in high grade glial tumors during temozolomide and radiotherapy. Int Immunopharmacol 14:546–549
Desjardins A, Reardon DA, Herndon JE 2nd, Marcello J, Quinn JA, Rich JN et al (2008) Bevacizumab plus irinotecan in recurrent WHO grade 3 malignant gliomas. Clin Cancer Res 14:7068–7073
Dolecek TA, Propp JM, Stroup NE, Kruchko C (2012) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro-Oncology 14(Suppl 5):v1–49
Franceschi E, Agati R, Brandes AA (2011) End points for Phase II trials in recurrent glioblastoma: the cornerstone for a new era. Expert Rev Anticancer Ther 11:1713–1717
Friedman HS, Petros WP, Friedman AH, Schaaf LJ, Kerby T, Lawyer J et al (1999) Irinotecan therapy in adults with recurrent or progressive malignant glioma. J Clin Oncol 17:1516–1525
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740
Gállego Pérez-Larraya J, Lahutte M, Petrirena G, Reyes-Botero G, González-Aguilar A, Houillier C et al (2012) Response assessment in recurrent glioblastoma treated with irinotecan-bevacizumab: comparative analysis of the Macdonald, RECIST, RANO, and RECIST + F criteria. Neuro-Oncology 14:667–673
Gil MJ, de Las Peñas R, Reynés G, Balañá C, Peréz-Segura P, García-Velasco A et al (2012) Bevacizumab plus irinotecan in recurrent malignant glioma shows high overall survival in a multicenter retrospective pooled series of the Spanish Neuro-Oncology Research Group (GEINO). Anticancer Drugs 23:659–665
Henriksson R, Asklund T, Poulsen HS (2011) Impact of therapy on quality of life, neurocognitive function and their correlates in glioblastoma multiforme: a review. J Neurooncol 104:639–646
Hofer S, Elandt K, Greil R, Hottinger AF, Huber U, Lemke D et al (2011) Clinical outcome with bevacizumab in patients with recurrent high-grade glioma treated outside clinical trials. Acta Oncol 50:630–635
Huse JT, Holland EC (2010) Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nat Rev Cancer 10:319–331
Kang TY, Jin T, Elinzano H, Peereboom D (2008) Irinotecan and bevacizumab in progressive primary brain tumors, an evaluation of efficacy and safety. J Neurooncol 89:113–118
Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I et al (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27:740–745
Lamszus K, Ulbricht U, Matschke J, Brockmann MA, Fillbrandt R, Westphal M (2003) Levels of soluble vascular endothelial growth factor (VEGF) receptor 1 in astrocytic tumors and its relation to malignancy, vascularity, and VEGF-A. Clin Cancer Res 9:1399–1405
Lu KV, Chang JP, Parachoniak CA, Pandika MM, Aghi MK, Meyronet D et al (2012) VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell 22:21–35
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Prados M, Cloughesy T, Samant M, Fang L, Wen PY, Mikkelsen T et al (2011) Response as a predictor of survival in patients with recurrent glioblastoma treated with bevacizumab. Neuro Oncol 13:143–151
Stark-Vance V (2005) Bevacizumab and CPT-11 in the treatment of relapsed malignant glioma. Presented at: World Federation of Neuro-Oncology Second Quadrennial Meeting. Edinburgh, Scotland, 4–7 May 2005 (Abstract 342)
Stefanik DF, Fellows WK, Rizkalla LR, Rizkalla WM, Stefanik PP, Deleo AB et al (2001) Monoclonal antibodies to vascular endothelial growth factor (VEGF) and the VEGF receptor, FLT-1, inhibit the growth of C6 glioma in a mouse xenograft. J Neurooncol 55:91–100
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L et al (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Marcello J, Reardon DA, Quinn JA et al (2007) Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 25:4722–4729
Vredenburgh JJ, Desjardins A, Reardon DA, Friedman HS (2009) Experience with irinotecan for the treatment of malignant glioma. Neuro Oncol 11:80–91
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Wick W, Weller M, van den Bent M, Stupp R (2010) Bevacizumab and recurrent malignant gliomas: a European perspective. J Clin Oncol 28:e188–e189
Wong ET, Hess KR, Gleason MJ, Jaeckle KA, Kyritsis AP, Prados MD et al (1999) Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 17:2572–2578
Xu T, Chen J, Lu Y, Wolff JE (2010) Effects of bevacizumab plus irinotecan on response and survival in patients with recurrent malignant glioma: a systematic review and survival-gain analysis. BMC Cancer 10:252
Yung WK, Albright RE, Olson J, Fredericks R, Fink K, Prados MD et al (2000) A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer 83:588–593
Conflict of interest
There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Demirci, U., Tufan, G., Aktas, B. et al. Bevacizumab plus irinotecan in recurrent or progressive malign glioma: a multicenter study of the Anatolian Society of Medical Oncology (ASMO). J Cancer Res Clin Oncol 139, 829–835 (2013). https://doi.org/10.1007/s00432-013-1390-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-013-1390-8