Abstract
Purposes
Trastuzumab is known to be effective for early and advanced stages of breast cancer but optimal duration for early-stage breast cancer (EBC) is not well known. We evaluated the efficacy and toxicity of 9- and 52-week trastuzumab therapy for EBC retrospectively.
Methods
In this multicenter study, the medical records of all patients with EBC were analyzed in 8 centers retrospectively. Totally consecutive, 479 female patients who received trastuzumab in the adjuvant treatment were evaluated for disease-free survival (DFS), overall survival (OS), efficacy, and toxicity.
Results
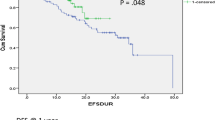
There were 181 (37.8 %) and 298 (62.2 %) patients in the 9- and 52-week trastuzumab groups, respectively. Median follow-up was 30.6 months (5.7–68.9) in the 9-week trastuzumab group and 29.3 months (5.9–59.6) in the 52-week trastuzumab group. Thirty-six month DFS was 90 and 85 % (P = 0.132) in the 9- and 52-week trastuzumab treatment groups, respectively, and 36-month OS was 96 and 97 % in the 9- and 52-week trastuzumab groups, respectively (P = 0.779). Symptomatic cardiotoxicity was observed in 1 (0.6 %) patient in the 9-week trastuzumab group and in 4 (1.3 %) patients in the 52-week trastuzumab group.
Conclusions
In this study, similar outcomes were found in the 9- and 52-week trastuzumab treatment groups.


Similar content being viewed by others
References
Boekhout AH, Beijnen JH, Schellens JH (2011) Trastuzumab. Oncologist 16:800–810
Chen T, Xu T, Li Y, Liang C, Chen J, Lu Y, Wu Z, Wu S (2006) Risk of cardiac dysfunction with trastuzumab in breast cancer patients: a meta-analysis. Cancer Treat Rev 37:312–320
Common Terminology Criteria for Adverse Events v3.0 (CTCAE) (2006) Publish Date: August 9, 2006. www.eortc.be/services/doc/ctc/ctcaev3.pdf. Accessed 2011 July 29
Costa RB, Kurra G, Greenberg L, Geyer CE (2010) Efficacy and cardiac safety of adjuvant trastuzumab-based chemotherapy regimens for HER2-positive early breast cancer. Ann Oncol 21:2153–2160
Ewer MS, Lippman SM (2005) Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J Clin Oncol 23:2900–2902
Gianni L, Dafni U, Gelber RD, Azambuja E, Muehlbauer S, Goldhirsch A, Untch M, Smith I, Baselga J, Jackisch C, Cameron D, Mano M, Pedrini JL, Veronesi A, Mendiola C, Pluzanska A, Semiglazov V, Vrdoljak E, Eckart MJ, Shen Z, Skiadopoulos G, Procter M, Pritchard KI, Piccart-Gebhart MJ, Bell R (2011) Herceptin Adjuvant (HERA) Trial Study Team Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol 12:236–244
Gonzalez-Angulo AM, Litton JK, Broglio KR, Meric-Bernstam F, Rakkhit R, Cardoso F, Peintinger F, Hanrahan EO, Sahin A, Guray M, Larsimont D, Feoli F, Stranzl H, Buchholz TA, Valero V, Theriault R, Piccart-Gebhart M, Ravdin PM, Berry DA, Hortobagyi GN (2009) High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol 27:5700–5706
Joensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R, Utriainen T, Kokko R, Hemminki A, Tarkkanen M, Turpeenniemi-Hujanen T, Jyrkkiö S, Flander M, Helle L, Ingalsuo S, Johansson K, Jääskeläinen AS, Pajunen M, Rauhala M, Kaleva-Kerola J, Salminen T, Leinonen M, Elomaa I, Isola J, FinHer Study Investigators (2006) Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med 354:809–820
Joensuu H, Bono P, Kataja V, Alanko T, Kokko R, Asola R, Utriainen T, Turpeenniemi-Hujanen T, Jyrkkiö S, Möykkynen K, Helle L, Ingalsuo S, Pajunen M, Huusko M, Salminen T, Auvinen P, Leinonen H, Leinonen M, Isola J, Kellokumpu-Lehtinen PL (2009) Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol 27:5685–5692
McArthur HL, Mahoney KM, Morris PG, Patil S, Jacks LM, Howard J, Norton L, Hudis CA (2011) Adjuvant trastuzumab with chemotherapy is effective in women with small, node-negative, HER2-positive breast cancer. Cancer 117:5461–5468
Paik S, Hazan R, Fisher ER, Sass RE, Fisher B, Redmond C, Schlessinger J, Lippman ME, King CR (1990) Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: prognostic significance of erbB-2 protein overexpression in primary breast cancer. J Clin Oncol 8:103–112
Perez EA, Romond EH, Suman VJ, Jeong JH, Davidson NE, Geyer CE Jr, Martino S, Mamounas EP, Kaufman PA, Wolmark N (2011a) Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol 29:3366–3373
Perez EA, Suman VJ, Davidson NE, Gralow JR, Kaufman PA, Visscher DW, Chen B, Ingle JN, Dakhil SR, Zujewski J, Moreno-Aspitia A, Pisansky TM, Jenkins RB (2011b) Sequential versus concurrent trastuzumab in adjuvant chemotherapy for breast cancer. J Clin Oncol 29:4491–4497
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Láng I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Rüschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353:1659–1672
Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353:1673–1684
Sawaki M, Tokudome N, Mizuno T, Nakayama T, Taira N, Bando H, Murakami S, Yamamoto Y, Kashiwaba M, Iwata H, Uemura Y, Ohashi Y (2011) Evaluation of trastuzumab without chemotherapy as a post-operative adjuvant therapy in HER2-positive elderly breast cancer patients: randomized controlled trial [RESPECT (N-SAS BC07)]. Jpn J Clin Oncol 41:709–712
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177–182
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244:707–712
Slamon D, Eiermann W, Robert N (2005) Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC/T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC/TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER-2 positive early breast cancer patients: BCIRG 006 study. Breast Cancer Res Treat 94(Suppl 1):S5a
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, Pinter T, Valero V, Liu MC, Sauter G, von Minckwitz G, Visco F, Bee V, Buyse M, Bendahmane B, Tabah-Fisch I, Lindsay MA, Riva A, Crown J (2011) Breast Cancer International Research Group. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 365:1273–1283
Spielmann M, Roché H, Delozier T, Canon JL, Romieu G, Bourgeois H, Extra JM, Serin D, Kerbrat P, Machiels JP, Lortholary A, Orfeuvre H, Campone M, Hardy-Bessard AC, Coudert B, Maerevoet M, Piot G, Kramar A, Martin AL, Penault-Llorca F (2009) Trastuzumab for patients with axillary-node-positive breast cancer: results of the FNCLCC-PACS 04 trial. J Clin Oncol 27:6129–6134
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Tonyali, O., Coskun, U., Sener, N. et al. Nine-week trastuzumab treatment versus 52-week trastuzumab treatment for HER2-positive early-stage breast cancer. J Cancer Res Clin Oncol 138, 2145–2151 (2012). https://doi.org/10.1007/s00432-012-1296-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-012-1296-x