Abstract
Purpose
p53 gene plays a crucial role in the response to therapy. Since it is inactivated in the majority of human cancers, it is strongly believed that the p53 mutations confer resistance to therapeutics. In this paper we analyzed the influence of two mechanistically diverse antitumor agents—cisplatin and methotrexate on the proliferation and cell cycle of two head and neck squamous cancer cell lines HEp-2 (wild type p53 gene, but HPV 18/E6-inactivated protein) and CAL 27 (mutated p53 gene), along with the influence of adenovirally mediated p53 overexpression in modulation of cisplatin and methoterexate effects, whereby subtoxic vector/compound concentrations were employed.
Methods
p53 gene was introduced into tumor cells using adenoviral vector (AdCMV-p53). The cell cycle perturbations were measured by two parameter flow cytometry. The expression of p53, p21WAF1/CIP1 and cyclin B1 proteins was examined using immunocytochemistry and western blot methods.
Results
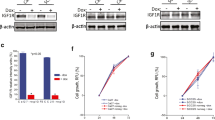
In CAL 27 cells overexpression of p53 completely abrogated high S phase content observed in methotrexate-treated cells into a G1 and slight G2 arrest, while it sustained G2 arrest of the cells treated with cisplatin, along with the reduction of DNA synthesis and cyclin B1 expression. On the other hand, in HEp-2 cell line p53 overexpression slightly slowed down the progression through S phase in cells treated with methotrexate, decreased the cyclin B1 expression only after 24 h, and failed to sustain the G2 arrest after treatment with cisplatin alone. Instead, it increased the population of S phase cells that were not actively synthesizing DNA, sustained cyclin B1 expression and allowed the G2 cells to progress through mitosis.
Conclusions
This study demonstrates that adenovirally mediated p53 overexpression at sub-cytotoxic levels enhanced the activity of low doses of cisplatin and methotrexate in HEp-2 and CAL 27 cells through changes in the cell cycle. However, the mechanisms of these effects differ depending on the genetic context and on the chemotherapeutics’ modality of action.










Similar content being viewed by others
References
Bhonde MR, Hanski M-L, Budczies J, Cao M, Gillissen B, Moorthy D, Simonetta F, Scherübl H, Truss M, Hagemeier C, Mewes H-W, Daniel PT, Zeitz M, Hanski C (2006) DNA damage-induced expression of p53 suppresses mitotic checkpoint kinase hMps1: the lack of this suppression in p53MUT cells contributes to apoptosis. J Biol Chem 281:8675–8685. doi:10.1074/jbc.M511333200
Blagosklonny MV (2002) p53: an ubiquitous target of anticancer drugs. Int J Cancer 98:161–166. doi:10.1002/ijc.10158
Brugarolas J, Moberg K, Boyd SD, Taya Y, Jacks T, Lees JA (1999) Inhibition of cyclin-dependent kinase 2 by p21 is necessary for retinoblastoma protein-mediated G1 arrest after gamma-irradiation. Proc Natl Acad Sci USA 96:1002–1007. doi:10.1073/pnas.96.3.1002
Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B (1998) Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282:1497–1501. doi:10.1126/science.282.5393.1497
Bunz F, Hwang PM, Torrance C, Waldmann T, Zhang Y, Dillehay L, Williams J, Lengauer C, Kinzler KW, Vogelstein B (1999) Disruption of p53 in human cancer cells aster s the responses to therapeutic agents. J Clin Invest 104:263–269. doi:10.1172/JCI6863
Chang DC, Xu N, Luo KQ (2003) Degradation of cyclin B is required for the onset of anaphase in mammalian cells. J Biol Chem 278:37865–37873. doi:10.1074/jbc.M306376200
Charrier-Savournin FB, Chateau M-T, Gire V, Sedivy J, Piette J, Dulić V (2004) p21-mediated nuclear retention of cyclin B1-Cdk1 in response to genotoxic stress. Mol Biol Cell 15:3965–3976. doi:10.1091/mbc.E03-12-0871
Clute P, Pines J (1999) Temporal and spatial control of cyclin B1 destruction in metaphase. Nat Cell Biol 1:82–87. doi:10.1038/10049
Dan S, Yamori T (2001) Repression of cyclin B1 expression after treatment with adriamycin, but not cisplatin in human lung cancer A549 cells. Biochem Biophys Res Commun 280:861–867. doi:10.1006/bbrc.2000.4231
el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75:817–825
Evan GI, Vousden KH (2001) Proliferation, cell cycle and apoptosis in cancer. Nature 411:342–348
Foulkes WD (2007) p53—master and commander. N Engl J Med 357:2539–2541
Fujiwara K, Daido S, Yamamoto A, Kobayashi R, Yokoyama T, Aoki H, Iwado E, Shinojima N, Kondo Y, Kondo S (2008) Pivotal role of the cyclin-dependent kinase inhibitor p21WAF1/CIP1 in apoptosis and autophagy. J Biol Chem 283:388–397
Funk JO, Waga S, Harry JB, Espling E, Stillman B, Galloway DA (1997) Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev 11:2090–2100
Gallagher WM, Brown R (1999) p53-oriented cancer therapies: current progress. Ann Oncol 10:139–150
Ganjavi H, Gee M, Narendran A, Parkinson N, Krishnamoorthy M, Freedman MH, Malkin D (2006) Adenovirus-mediated p53 gene therapy in osteosarcoma cell lines: sensitization to cisplatin and doxorubicin. Cancer Gene Ther 13:415–419
Gartel AL, Tyner AL (2002) The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther 1:639–649
Hait WN, Yang J-M (2006) The individualization of cancer therapy: the unexpected role of p53. Trans Am Clin Climatol Assoc 117:85–101
Hattangadi DK, DeMasters GA, Walker TD, Jones KR, Di X, Newsham IF, Gewirtz DA (2004) Influence of p53 and caspase 3 activity on cell death and senescence in response to methotrexate in the breast tumor cell. Biochem Pharmacol 68:1699–1708
Hollstein M, Shomer B, Greenblatt M, Soussi T, Hovig E, Montesano R, Harris CC (1996) Somatic point mutations in the p53 gene of human tumors and cell lines: updated compilation. Nucleic Acid Res 24:141–146
Horio Y, Hasegawa Y, Sekido Y, Takahashi M, Roth JA, Shimokata K (2000) Synergistic effects of adenovirus expressing wild-type p53 on chemosensitivity of non-small cell lung cancer cells. Cancer Gene Therapy 7:537–544
Horowitz J-A (1999) Adenovirus-mediated p53 gene therapy: overview of preclinical studies and potential clinical applications. Curr Opin Mol Ther 1:500–509
Innocente SA, Abrahamson JLA, Cogswell JP, Lee JM (1999) p53 regulates a G2 checkpoint through cyclin B1. Proc Natl Acad Sci 96:2147–2152
Inoue A, Narumi K, Matsubara N, Sugawara S, Saijo Y, Satoh K, Nukiwa T (2000) Administration of wild-type 53 adenoviral vector synergistically enhances the cytotoxicity of anti-cancer drugs in human lung cancer cells irrespective of the status of p53 gene. Cancer Lett 157:105–112
Jänicke RU, Sohn D, Schulze-Osthoff K (2008) The dark side of a tumor suppressor: anti-apoptotic p53. Cell Death Differ 15:959–976
Kandioler D, Stamatis G, Eberhardt W, Kappel S, Zöchbauer-Müller S, Kührer I, Mittlböck M, Zwrtek R, Aigner C, Bichler C, Tichy V, Hudec M, Bachleitner T, End A, Müller MR, Roth E, Klepetko W (2008) Growing clinical evidence for the interaction of the p53 genotype and response to induction chemotherapy in advanced non-small cell lung cancer. J Thorac Cardiovasc Surg 135:1036–1041
Kastan MB (2007) Wild-type p53: tumors can’t stand it. Cell 128:837–840
Kralj M, Pavelic J (2003) p21WAF1/CIP1 is more effective than p53 in growth suppression of mouse renal carcinoma cell line Renca in vitro and in vivo. J Cancer Res Clin Oncol 129:463–471
Kralj M, Husnjak K, Körbler T, Pavelic J (2003) Endogenous p21WAF1/CIP1 status predicts the response of human tumor cells to wild-type p53 and p21WAF1/CIP1 overexpression. Cancer Gene Ther 10:457–467
Kraljevic Pavelic S, Cacev T, Kralj M (2008) A dual role of p21waf1/cip1 gene in apoptosis of HEp-2 treated with cisplatin or methotrexate. Cancer Gene Ther 15:576–590
Krause K, Wasner M, Reinhard W, Haugwith U, Lange-ze Dohna C, Mossner J, Engeland K (2000) The tumor suppressor protein p53 can repress transcription of cyclin B. Nucl Acid Res 28:4410–4418
Kupryjanczyk J, Kraszewska E, Ziolkowska-Seta I, Madry R, Timorek A, Markowska J, Stelmachow J, Bidzinski M, Polish Ovarian Cancer Study Group (POCSG) (2008) TP53 status and taxane-platinum versus platinum-based therapy in ovarian cancer patients: a non-randomized retrospective study. BMC Cancer 8:27
LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, Fattaey A, Harlow E (1997) New functional activities for the p21 family of CDK inhibitors. Genes Dev 11:847–862
Lee SI, Brown MK, Eastman A (1999) Comparison of the efficacy of 7-hydroxystaurosporine (UCN-01) and other staurosporine analogs to abrogate cisplatin-induced cell cycle arrest in human breast cancer cell lines. Biochem Pharmacol 58:1713–1721
Linke SP, Clarkin KC, Di Leonardo A, Tsou A, Wahl GM (1996) A reversible, p53-dependent G0/G1 cell cycle arrest induced by ribonucleotide deplenon in the absence of detectable DNA damage. Genes Dev 10:934–947
Maeda T, Chong MT, Espino RA, Chua PP, Cao JQ, Chomey EG, Luong L, Tron VA (2002) Role of p21Waf-1 in regulating the G1 and G2/M checkpoints in ultraviolet-irradiated keratinocytes. J Invest Dermatol 119:513–521
Meek DW (2004) The p53 response to DNA damage. DNA Repair 3:1049–1056
Nielsen LL, Maneval DC (1998) p53 tumor suppressor gene therapy for cancer. Cancer Gene Ther 5:52–63
Peng Z (2005) Current status of gendicine in China: recombinant human Ad-p53 agent for treatment of cancers. Hum Gene Ther 16:1016–1027
Piaggio G, Farina A, Perrotti D, Manni I, Fuschi P, Sacchi A, Gaetano C (1995) Structure and growth-dependent regulation of the human cyclin B1 promoter. Exp Cell Res 216:396–402
Pines J, Hunter T (1989) Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell 58:833–846
Radhakrishnan SK, Feliciano CS, Najmabadi F, Haegebarth A, Kandel ES, Tyner AL, Gartel AL (2004) Constitutive expression of E2F-1 leads to p21-dependent cell cycle arrest in S phase of the cell cycle. Oncogene 23:4173–4176
Rakitina TV, Vasilevskaya IA, O’Dwyer PJ (2007) Inhibition of G1/S transition potentiates oxaliplatin-induced cell death in colon cancer cells lines. Biochem Pharmacol 73:1715–1726
Roth RA (2006) Adenovirus p53 gene therapy. Expert Opin Biol Ther 6:55–61
Slee EA, O’Connor DJ, Lu X (2004) To die or not to die: how does p53 decide? Oncogene 23:2809–2818
Sun D, Urrabaz R, Buzello C, Nguyen M (2002) Effects of cisplatin on expression of DNA ligases in MiaPaCa human pancreatic cancer cells. Biochem Biophys Res Commun 298:537–544
Supek F, Kralj M, Marjanović M, Šuman L, Šmuc T, Krizmanić I, Žinić B (2008) Atypical cytostatic mechanism of N-1-sulfonylcytosine derivatives determined by in vitro screening and computational analysis. Invest New Drugs 26(2):97–110
Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, Newman J, Reczek EE, Weissleder R, Jacks T (2007) Restoration of p53 function leads to tumour regression in vivo. Nature 445:661–665
Vousden KH (2006) Outcomes of p53 activation-spoilt for choice. J Cell Sci 119:5015–5020
Waldman T, Kinzler KW, Vogelstein B (1995) p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res 55:5187–5190
Wallace-Brodeur RR, Lowe SW (1999) Clinical implications of p53 mutations. Cell Mol Life Sci 55:64–75
Weinrib L, Li J-H, Donovan J, Huang D, Liu F-F (2001) Cisplatin chemotherapy plus adenoviral p53 gene therapy in EBV-positive and -negative nasopharyngeal carcinoma. Cancer Gene Ther 8:352–360
Yip HT, Chopra R, Chakrabarti R, Veena MS, Ramamurthy B, Srivatsan ES, Wang MB (2006) Cisplatin-induced growth arrest of head and neck cancer cells correlates with increased expression of p16 and p53. Arch Otolarynog Head Neck Surg 132:317–326
Zhou B-B, Bartek J (2004) Targeting the checkpoint kinases: chemosensitization versus chemoprotection. Nat Rev Cancer 4:1–10
Acknowledgments
Support for this study by the Ministry of Science, Education and Sport of Croatia is gratefully acknowledged (Projects 098-0982464-2393, 098-0982464-2514). The authors are particularly thankful to Dr. Anamaria Brozović for exceptionally useful discussions and experimental help and Dr. Ksenija Zahradka for providing us with the BrdU chemical and anti-BrdU antibody.
Author information
Authors and Affiliations
Corresponding author
Additional information
S. Kraljević Pavelić and M. Marjanović contributed equally to the preparation of this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kraljević Pavelić, S., Marjanović, M., Poznić, M. et al. Adenovirally mediated p53 overexpression diversely influence the cell cycle of HEp-2 and CAL 27 cell lines upon cisplatin and methotrexate treatment. J Cancer Res Clin Oncol 135, 1747–1761 (2009). https://doi.org/10.1007/s00432-009-0621-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-009-0621-5