Abstract
Purpose
Lysophosphatidic acid (LPA) is a multifunctional lipid mediator involved in triggering tumor cell invasion and metastasis, as well as malignant cell growth. LPA is also known to modulate the colony scattering of epithelial cancers, which is a prerequisite for cell invasion. However, the underlying details of how this is accomplished are not clear. Here we have investigated the roles of specific LPA receptor subtypes in cell scattering.
Methods
Gastrointestinal carcinoma cell lines were examined for cell scattering activity in response to LPA, and the expression of LPA receptor subtypes was determined by RT-PCR. The effect of down regulation of each LPA receptor in DLD1 cells was determined using a shRNA-lentivirus system. In addition, the effect of overexpression of LPA receptors on cell scattering was investigated using lentivirus expression constructs.
Results
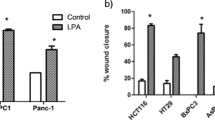
The colonies of AGS and DLD1, but not MKN74, cells were dispersed in response to LPA. RT-PCR analysis revealed that the mRNAs of LPA1, LPA2, and LPA3 were present in AGS and DLD1 cells, but only LPA2 mRNA was detected in MKN74 cells. In DLD1 cells, the scattering activity induced by LPA was partially blocked by pretreatment with PP2 and PD98059, inhibitors of src kinase and MEK, respectively. LPA1 knockdown with shRNA decreased the degree of cell scattering induced by LPA. Knockdown of LPA2 or LPA3 had no effect on LPA-induced scattering. In addition, overexpression of LPA1 in DLD1 cells slightly decreased the response time of LPA-induced cell scattering. On the contrary, MKN74 cells expressing exogenous LPA1 did not respond to LPA by scattering.
Conclusion
These results demonstrate that LPA1 mediates LPA-stimulated cell scattering of gastrointestinal carcinomas, but that activation of other intracellular pathways, besides those contributing to ERK phosphorylation, is also necessary for cell scattering in response to LPA.






Similar content being viewed by others
Abbreviations
- LPA:
-
Lysophosphatidic acid
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- shRNA:
-
Short hairpin ribonucleic acid
References
Anliker B, Chun J (2004) Lysophospholipid G protein-coupled receptors. J Biol Chem 279:20555–20558
Daaka Y (2002) Mitogenic action of LPA in prostate. Biochim Biophys Acta 1582:265–269
Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L (1998) A third-generation lentivirus vector with a conditional packaging system. J Virol 72:8463–8471
Goetzl EJ, An S (1998) Diversity of cellular receptors and functions for the lysophospholipid growth factors lysophosphatidic acid and sphingosine 1-phosphate. FASEB J 12:1589–1598
Hama K, Aoki J, Fukaya M, Kishi Y, Sakai T, Suzuki R, Ohta H, Yamori T, Watanabe M, Chun J, Arai H (2004) Lysophosphatidic acid and autotaxin stimulate cell motility of neoplastic and non-neoplastic cells through LPA1. J Biol Chem 279:17634–17639
Hao F, Tan M, Xu X, Han J, Miller DD, Tigyi G, Cui MZ (2007) Lysophosphatidic acid induces prostate cancer PC3 cell migration via activation of LPA(1), p42 and p38alpha. Biochim Biophys Acta 1771:883–892
Huang RY, Wang SM, Hsieh CY, Wu JC (2008) Lysophosphatidic acid induces ovarian cancer cell dispersal by activating Fyn kinase associated with p120-catenin. Int J Cancer (in press)
Huber MA, Kraut N, Beug H (2005) Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol 17:548–558
Hwang JI, Fraser ID, Choi S, Qin XF, Simon MI (2004) Analysis of C5a-mediated chemotaxis by lentiviral delivery of small interfering RNA. Proc Natl Acad Sci USA 101:488–493
Ishii I, Fukushima N, Ye X, Chun J (2004) Lysophospholipid receptors: signaling and biology. Annu Rev Biochem 73:321–354
Jourquin J, Yang N, Kam Y, Guess C, Quaranta V (2006) Dispersal of epithelial cancer cell colonies by lysophosphatidic acid (LPA). J Cell Physiol 206:337–346
Khurana S, Tomar A, George SP, Wang Y, Siddiqui MR, Guo H, Tigyi G, Mathew S (2008) Autotaxin and lysophosphatidic acid stimulate intestinal cell motility by redistribution of the actin modifying protein villin to the developing lamellipodia. Exp Cell Res 314:530–542
Larue L, Bellacosa A (2005) Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3’ kinase/AKT pathways. Oncogene 24:7443–7454
Lee CW, Rivera R, Gardell S, Dubin AE, Chun J (2006) GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J Biol Chem 281:23589–23597
Long IS, Han K, Li M, Shirasawa S, Sasazuki T, Johnston M, Tsao MS (2003) Met receptor overexpression and oncogenic Ki-ras mutation cooperate to enhance tumorigenicity of colon cancer cells in vivo. Mol Cancer Res 1:393–401
Mills GB, Moolenaar WH (2003) The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer 3:582–591
Mills GB, May C, Hill M, Campbell S, Shaw P, Marks A (1990) Ascitic fluid from human ovarian cancer patients contains growth factors necessary for intraperitoneal growth of human ovarian adenocarcinoma cells. J Clin Invest 86:851–855
Moolenaar WH (1995) Lysophosphatidic acid, a multifunctional phospholipid messenger. J Biol Chem 270:12949–12952
Shah BH, Baukal AJ, Shah FB, Catt KJ (2005) Mechanisms of extracellularly regulated kinases 1/2 activation in adrenal glomerulosa cells by lysophosphatidic acid and epidermal growth factor. Mol Endocrinol 19:2535–2548
Shida D, Kitayama J, Yamaguchi H, Okaji Y, Tsuno NH, Watanabe T, Takuwa Y, Nagawa H (2003) Lysophosphatidic acid (LPA) enhances the metastatic potential of human colon carcinoma DLD1 cells through LPA1. Cancer Res 63:1706–1711
Thiery JP (2002) Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2:442–454
Tokumura A, Majima E, Kariya Y, Tominaga K, Kogure K, Yasuda K, Fukuzawa K (2002) Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem 277:39436–39442
Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J, Arai H (2002) Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol 158:227–233
Xie Y, Gibbs TC, Mukhin YV, Meier KE (2002) Role for 18:1 lysophosphatidic acid as an autocrine mediator in prostate cancer cells. J Biol Chem 277:32516–32526
Xu Y, Shen Z, Wiper DW, Wu M, Morton RE, Elson P, Kennedy AW, Belinson J, Markman M, Casey G (1998) Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. Jama 280:719–723
Yang M, Zhong WW, Srivastava N, Slavin A, Yang J, Hoey T, An S (2005) G protein-coupled lysophosphatidic acid receptors stimulate proliferation of colon cancer cells through the β-catenin pathway. Proc Natl Acad Sci USA 102:6027–6032
Yee JK, Friedmann T, Burns JC (1994) Generation of high-titer pseudotyped retroviral vectors with very broad host range. Methods Cell Biol 43(Pt A):99–112
Yun CC, Sun H, Wang D, Rusovici R, Castleberry A, Hall RA, Shim H (2005) LPA2 receptor mediates mitogenic signals in human colon cancer cells. Am J Physiol Cell Physiol 289:C2–C11
Acknowledgments
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD)(KRF- 2007-331-C00232) and by the Korea University Grants.
Author information
Authors and Affiliations
Corresponding author
Additional information
Y. L. Kim and K.-J. Shin equally contributed to this work.
Rights and permissions
About this article
Cite this article
Shin, KJ., Kim, Y.L., Lee, S. et al. Lysophosphatidic acid signaling through LPA receptor subtype 1 induces colony scattering of gastrointestinal cancer cells. J Cancer Res Clin Oncol 135, 45–52 (2009). https://doi.org/10.1007/s00432-008-0441-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-008-0441-z