Abstract
Purpose
SCC-112 is a novel cell cycle-related gene and differentially expressed in cancers. Suggesting the complex role of SCC-112 might be existent in cell proliferation and tumor development. The relative research on SCC-112 has been few so far. This study is attempted to explore the role of SCC-112 in tumorigenesis.
Experimental design
RT-PCR and western blot were performed on seven tumor-normal paired tissues and nine cell lines. Immunohistochemistry was carried out for analyzing the expression of SCC-112 in nasopharyngeal tissues. 293T and three nasopharyngeal cell lines were transfected with expression vector (pCMV-SPORT6-SCC-112) or its siRNA. Cell proliferation was examined by MTT and clone formation experiments. Immunoprecipitation determined the interacted protein of SCC-112, and FACS detected cell cycle parameter on cells treated with synchronized reagent.
Results
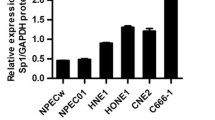
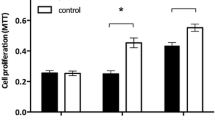
SCC-112 (∼150 kDa) is up-regulated in tumor tissue as compared to the corresponding normal tissue and was detected in the tested cell lines. Overexpression of SCC-112 (∼150 kDa) in 293T and three nasopharyngeal cell lines promoted cell proliferation and clone formation while downregulation of SCC-112 (∼150 kDa) in these cells resulted in the opposite. Moreover, SCC-112 was found to interact with p63 and overexpression of SCC-112 up-regulated p63 expression. SCC-112 expression level positively correlated with cells in G2/M phase.
Conclusions
These findings suggest that SCC-112 improve cell proliferation and contributes to tumorigenesis by interacting with p63 and promoting cell cycling. SCC-112 might be an alternative target in tumor biomarking and mechanistic investigation.






Similar content being viewed by others
References
Bakal CJ, Finan D, LaRose J, Wells CD, Gish G, Kulkarni S, DeSepulveda P, Wilde A, Rottapel R (2005) The Rho GTP exchange factor Lfc promotes spindle assembly in early mitosis. Proc Natl Acad Sci USA 102:9529–9534
Bakiri L, Lallemand D, Bossy-Wetzel E, Yaniv M (2000) Cell cycle-dependent variations in c-Jun and JunB phosphorylation: a role in the control of cyclin D1 expression. Embo J 19:2056–2068
Bar-Sagi D, Hall A (2000) Ras and Rho GTPases: a family reunion. Cell 103:227–238
Bar J, Lukaschuk N, Zalcenstein A, Wilder S, Seger R, Oren M (2005) The PI3K inhibitor LY294002 prevents p53 induction by DNA damage and attenuates chemotherapy-induced apoptosis. Cell Death Differ 12:1578–1587
Barbieri CE, Barton CE, Pietenpol JA (2003) Delta Np63 alpha expression is regulated by the phosphoinositide 3-kinase pathway. J Biol Chem 278:51408–51414
Baroffio A, Dupin E, Le Douarin NM (1988) Clone-forming ability and differentiation potential of migratory neural crest cells. Proc Natl Acad Sci USA 85:5325–5329
Benitah SA, Valeron PF, van Aelst L, Marshall CJ, Lacal JC (2004) Rho GTPases in human cancer: an unresolved link to upstream and downstream transcriptional regulation. Biochim Biophys Acta 1705:121–132
Coleman ML, Marshall CJ, Olson MF (2004) RAS and RHO GTPases in G1-phase cell-cycle regulation. Nat Rev Mol Cell Biol 5:355–366
Crook T, Nicholls JM, Brooks L, O’Nions J, Allday MJ (2000) High level expression of deltaN-p63: a mechanism for the inactivation of p53 in undifferentiated nasopharyngeal carcinoma (NPC)? Oncogene 19:3439–3444
Davis LD, Zhang W, Merseburger A, Young D, Xu L, Rhim JS, Moul JW, Srivastava S, Sesterhenn IA (2002) p63 expression profile in normal and malignant prostate epithelial cells. Anticancer Res 22:3819–3825
Di Como CJ, Urist MJ, Babayan I, Drobnjak M, Hedvat CV, Teruya-Feldstein J, Pohar K, Hoos A, Cordon-Cardo C (2002) p63 expression profiles in human normal and tumor tissues. Clin Cancer Res 8:494–501
Eferl R, Wagner EF (2003) AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer 3:859–868
Evan GI, Vousden KH (2001) Proliferation, cell cycle and apoptosis in cancer. Nature 411:342–348
Feng BJ, Huang W, Shugart YY, Lee MK, Zhang F, Xia JC, Wang HY, Huang TB, Jian SW, Huang P (2002) Genome-wide scan for familial nasopharyngeal carcinoma reveals evidence of linkage to chromosome 4. Nat Genet 31:395–399
Fukada T, Ohtani T, Yoshida Y, Shirogane T, Nishida K, Nakajima K, Hibi M, Hirano T (1998) STAT3 orchestrates contradictory signals in cytokine-induced G1 to S cell-cycle transition. Embo J 17:6670–6677
Geradts J, Ingram CD (2000) Abnormal expression of cell cycle regulatory proteins in ductal and lobular carcinomas of the breast. Mod Pathol 13:945–953
Glickman JN, Yang A, Shahsafaei A, McKeon F, Odze RD (2001) Expression of p53-related protein p63 in the gastrointestinal tract and in esophageal metaplastic and neoplastic disorders. Hum Pathol 32:1157–1165
Guo C, Pan ZG, Li DJ, Yun JP, Zheng MZ, Hu ZY, Cheng LZ, Zeng YX (2006) The expression of p63 is associated with the differential stage in nasopharyngeal carcinoma and EBV infection. J Transl Med 4:23
Jamerson MH, Johnson MD, Korsmeyer SJ, Furth PA, Dickson RB (2004) Bax regulates c-Myc-induced mammary tumour apoptosis but not proliferation in MMTV-c-myc transgenic mice. Br J Cancer 91:1372–1379
Knosel T, Petersen S, Schwabe H, Schluns K, Stein U, Schlag PM, Dietel M, Petersen I (2002) Incidence of chromosomal imbalances in advanced colorectal carcinomas and their metastases. Virchows Arch 440:187–194
Kumar D, Sakabe I, Patel S, Zhang Y, Ahmad I, Gehan EA, Whiteside TL, Kasid U (2004) SCC-112, a novel cell cycle-regulated molecule, exhibits reduced expression in human renal carcinomas. Gene 328:187–196
Kurada P, White K (1998) Ras promotes cell survival in Drosophila by downregulating hid expression. Cell 95:319–329
Kwan KM, Kirschner MW (2005) A microtubule-binding Rho-GEF controls cell morphology during convergent extension of Xenopus laevis. Development 132:4599–4610
Massion PP, Taflan PM, Jamshedur Rahman SM, Yildiz P, Shyr Y, Edgerton ME, Westfall MD, Roberts JR, Pietenpol JA, Carbone DP, Gonzalez AL (2003) Significance of p63 amplification and overexpression in lung cancer development and prognosis. Cancer Res 63:7113–7121
Mo H, Vita M, Crespin M, Henriksson M (2006) Myc overexpression enhances apoptosis induced by small molecules. Cell Cycle 5:2191–2194
Nagata K, Inagaki M (2005) Cytoskeletal modification of Rho guanine nucleotide exchange factor activity: identification of a Rho guanine nucleotide exchange factor as a binding partner for Sept9b, a mammalian septin. Oncogene 24:65–76
Obaya AJ, Mateyak MK, Sedivy JM (1999) Mysterious liaisons: the relationship between c-Myc and the cell cycle. Oncogene 18:2934–2941
Okkenhaug K, Vanhaesebroeck B (2003) PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol 3:317–330
Pan ZG, Kashuba VI, Liu XQ, Shao JY, Zhang RH, Jiang JH, Guo C, Zabarovsky E, Ernberg I, Zeng YX (2005) High frequency somatic mutations in RASSF1A in nasopharyngeal carcinoma. Cancer Biol Ther 4:1116–1122
Park BJ, Lee SJ, Kim JI, Lee SJ, Lee CH, Chang SG, Park JH, Chi SG (2000) Frequent alteration of p63 expression in human primary bladder carcinomas. Cancer Res 60:3370–3374
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA (2000) Molecular portraits of human breast tumours. Nature 406:747–752
Qiu RG, Chen J, Kirn D, McCormick F, Symons M (1995) An essential role for Rac in Ras transformation. Nature 374:457–459
Radtke F, Raj K (2003) The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer 3:756–767
Repasky GA, Chenette EJ, Der CJ (2004) Renewing the conspiracy theory debate: does Raf function alone to mediate Ras oncogenesis? Trends Cell Biol 14:639–647
Rossman KL, Der CJ, Sondek J (2005) GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 6:167–180
Roux P, Gauthier-Rouviere C, Doucet-Brutin S, Fort P (1997) The small GTPases Cdc42Hs, Rac1 and RhoG delineate Raf-independent pathways that cooperate to transform NIH3T3 cells. Curr Biol 7:629–637
Signoretti S, Waltregny D, Dilks J, Isaac B, Lin D, Garraway L, Yang A, Montironi R, McKeon F, Loda M (2000) p63 is a prostate basal cell marker and is required for prostate development. Am J Pathol 157:1769–1775
Sun J, Wiklund F, Zheng SL, Chang B, Balter K, Li L, Johansson JE, Li G, Adami HO, Liu W (2005) Sequence variants in Toll-like receptor gene cluster (TLR6-TLR1-TLR10) and prostate cancer risk. J Natl Cancer Inst 97:525–532
Wang S, Lloyd RV, Hutzler MJ, Safran MS, Patwardhan NA, Khan A (2000) The role of cell cycle regulatory protein, cyclin D1, in the progression of thyroid cancer. Mod Pathol 13:882–887
Whitfield ML, Sherlock G, Saldanha AJ, Murray JI, Ball CA, Alexander KE, Matese JC, Perou CM, Hurt MM, Brown PO, Botstein D (2002) Identification of genes periodically expressed in the human cell cycle and their expression in tumors. Mol Biol Cell 13:1977–2000
Acknowledgments
This work was supported by The National Natural Science Foundation of China (No. 30570817) and State Key Basic Research Programs 973 of China (NO.2002CB513101 and No.2004CB518701). We thank Ying Huang for collecting tissues and Zhong-yuan Tian for helping data management.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, M.Z., Zheng, L.M. & Zeng, Y.X. SCC-112 gene is involved in tumor progression and promotes the cell proliferation in G2/M phase. J Cancer Res Clin Oncol 134, 453–462 (2008). https://doi.org/10.1007/s00432-007-0306-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-007-0306-x