Abstract
Purpose
The change of cell mobility is one of the preconditions of tumor metastasis. Cell skeleton alteration and rearrangement of F-actin was closely related to cell mobility. Ezrin is a membrane-cytoskeleton organizer that can mediate the rearrangement and the function of F-actin. In this paper, we investigated the effect of ezrin on hepatocellullar carcinoma cell growth and invasiveness.
Methods
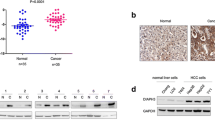
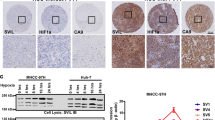
Hepatocellular carcinoma cell lines such as MHCC-1, MHCC97-H, SF7721, SMMC7721, Hep3B, and HepG2 were chosen in this study. We first examined the expression and the distribution of ezrin and F-actin in these cell lines using immunofluorescence, RT–PCR, and the western blot. Next we used small interfering RNA (siRNA) to down-regulate ezrin expression in MHCC-1, MHCC97-H, SF7721, and HepG2 to investigate the role of ezrin in tumor cell growth and invasiveness.
Results
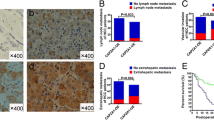
Our preliminary results showed that the expression of ezrin and γ-actin in MHCC-1, MHCC97-H, and SF7721 with higher metastatic potential were obviously up-regulated than those in SMMC7721, Hep3B, and HepG2 with lower potential. No different expression of β-actin was found in the above tumor cell lines. The outcome of RNAi indicated that decreasing ezrin expression can notably inhibit the proliferation of the four hepatocellular carcinoma cell lines (p < 0.01, n = 10). The proportion of cells in G2-M phase also decreased after RNAi. The number of pseudopods decreased as well after RNAi treatment (p < 0.01, n = 5). The mobility and invasiveness of cancer cells decreased with decreasing ezrin expression tested by transwell assay (p < 0.01, n = 8).
Conclusion
Ezrin plays an important role in the process of hepatocellular carcinoma cell proliferation, migration, and invasiveness.










Similar content being viewed by others
References
Andreoli C, Martin M, Le Borgne R, Reggio H, Mangeat P (1994). Ezrin has properties to self-associate at the plasma membrane. J Cell Sci 107(9):2509–2521
Bretscher A, Edwards K, Fehon RG (2002) ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol 3:586–599
Chen ZC, Fadiel A, Feng YJ, Ohtani K, Rutherford T, Naftolin F (2001) Ovarian epithelial carcinoma tyrosine phosphorylation, cell proliferation, and ezrin translocation are stimulated by interleukin 1 alpha and epidermal growth factor. Cancer 92:3068–3075
Crepaldi T, Gautreau A, Comoglio PM, Louvard D, Arpin M (1997) Ezrin is an effector of hepatocyte growth factor-mediated migration and morphogenesis in epithelial cell. J Cell Biol 138(2):423–434
Harrision GM, Davies G, Martin TA (2002) Distribution and expression of CD44 isoforms and ezrin during prostate cancer–endothelium interaction. Int J Oncol 21:935–940
Hiscox S, Jiang WG (1999) Ezrin regulates cell–cell and cell–matrix adhesion: a possible role with E-cadherin/beta-catenin. J Cell Sci 112(18):3081–3090
Kaul SC, Mitsui Y, Komatsu Y, Reddel RR, Wadhwa R (1996) A highly expressed 81 kDa protein in immortalized mouse fibroblast: its proliferative function and identity with ezrin. Oncogene 13(6):1231–1237
Khanna C, Wan X, Bose S, Cassaday R, Olomu O, Mendoza A, Yeung C, Gorlick R, Hewitt SM, Helman LJ (2004) The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med 10(2):182–186
Krieg J, Hunter T (1992) Identification of the two major epidermal growth factor-induced tyrosine phosphorylation sites in the microvillar core protein ezrin. J Biol Chem 267:19258–19265
Lamb RF, Ozanne BW, Roy C, McGarry L, Stipp C, Mangeat P, Jay DG (1997) Essential functions of ezrin in maintenance of cell shape and lamellipodial extension in normal and transformed fibroblasts. Curr Biol 7(9):682–688
Masahide T, Toshiro N, Yukihito S (2000) Altered expression of the ERM proteins in lung adenocarcinoma. US Can Acad Pathol 80(11):1643–1650
Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S, Tsukita S (1998) Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol 140:647–657
Nguyen R, Reczek D, Bretscher A (2001) Hierarchy of merlin and ezrin N and C-terminal domain interactions in homo and heterotypic associations and their relationship to binding of scaffolding proteins EBP50 and E3KARP. J Biol Chem 276:7621–7629
Ohtani K, Sakamoto H, Rutherford T, Chen Z, Satoh K, Naftolin F (1999) Ezrin, a membrane-cytoskeletal linking protein, is involved in the process of invasion of endometrial cancer cells. Cancer Lett 147(1/2):31–38
Pawlak G, Helfman DM (2000) Cytoskeletal changes in cell transformation and tumorigenesis. Curr Opin Genet Dev 11(1):41–47
Sainio M, Zhao F, Heiska L, Turunen O, den Bakker M, Zwarthoff E, Lutchman M, Rouleau GA, Jaaskelainen J, Vaheri A, Carpen O (1997) Neurofibromatosis 2 tumour suppressor protein colocalizes with ezrin and CD44 and associates with actin-containing cytoskeleton. J Cell Sci 110:2249–2260
Shen ZY, Xu LY, Chen MH, Li EM, Li JT, Wu XY, Zeng Y, Xu LY, Chen MH, et al. (2003) Upregulated expression of ezrin and invasive phenotype in malignantly transformed esophageal epithelial cells. World J Gastroenterol 9(6):1182–1186
Small JV (1994) Lamellipodia architecture: actin filament turnover and the lateral flow of actin filaments during motility. Semin Cell Biol 5:157–163
Small JV, Rottner K, Kaverina I, Anderson KI (1998) Assembling an actin cytoskeleton for cell attachment and movement. Biochim Biophys Acta 1404:271–281
Small JV, Stradal T, Vignal E, Rottner K (2002) The lamellipodium: where motility begins. Trends Cell Biol 12:112–120
Tran Quang C, Gautreau A, Arpin M, Treisman R (2000) Ezrin function is required for ROCK-mediated fibroblast transformation by the net and dbl oncogenes. EMBO J 19:4565–4576
Xie Q, Liu KD, Hu MY, Zhou K (2001) SF/HCF-c-met autocrine and paracrine promote metastasis in hepatocellular carcinoma. World J Gastroenterol 7:816–820
Yonemura S, Tsukita S, Tsukita S (1999) Direct involvement of ezrin/radixin/moesin (ERM)-binding membrane proteins in the organization of microvilli in collaboration with activated ERM proteins. J Cell Biol 145(7):1497–1509
Yonemura S, Matsui T, Tsukita S (2002) Rho-dependent and -independent activation mechanisms of ezrin/radixin/moesin proteins: an essential role for polyphosphoinositides in vivo. J Cell Sci 115:2569–2580
Yu Y, Khan J, Khanna C, Helman L, Meltzer PS, Merlino G (2004) Expression profiling identifies the cytoskeletal organizer ezrin and the developmental homeoprotein Six-1 as key metastasic regulators. Nat Med 10(2):175–181
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Hu, MY., Wu, WZ. et al. The membrane-cytoskeleton organizer ezrin is necessary for hepatocellular carcinoma cell growth and invasiveness. J Cancer Res Clin Oncol 132, 685–697 (2006). https://doi.org/10.1007/s00432-006-0117-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-006-0117-5