Abstract
Purpose
To investigate the anticancer activity and mode of action of butyroyloxymethyl-diethyl phosphate (AN-7), a prodrug of butyric acid and formaldehyde, as a single agent and in combination with doxorubicin in human carcinoma MCF-7 and the multidrug resistant MCF-7 Dx cell lines.
Methods
The anti-cancer activity of AN-7 as a single agent or in combination with doxorubicin was measured by the Hoechst cell viability and colony forming assays as well as by FACS analyses of cells stained with propidium iodide and annexin V-FITC. Modulations of protein expression and acetylation were measured by Western blot analyses. The number of doxorubicin–DNA adducts formed was evaluated using 14C-labeled doxorubicin.
Results
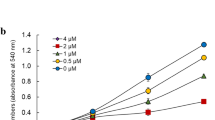
The AN-7 and homologous prodrugs exhibited similar growth inhibition effects against drug resistant and sensitive cells, and elicited their anticancer effect partially by inhibition of HDAC. The AN-7 transiently augmented histone acetylation and increase of p21 expression. Synergy between AN-7 and doxorubicin was demonstrated in the sensitive and the resistant cell lines by viability and colony formation assays and was further confirmed by FACS analysis showing an increase in cell mortality. The number of doxorubicin–DNA adducts in total genomic DNA isolated from cells treated with 14C-labeled doxorubicin and AN-7 increased substantially compared to treatment with doxorubicin only. Treatment with AN-7 or doxorubicin increased p53 acetylation that was further potentiated by their combination.
Conclusion
The AN-7 combined with doxorubicin overcame drug resistance; at least in part by the intracellularly releasable formaldehyde that augmented formation of doxorubicin–DNA adducts and butyric acid that induced histone and p53 acetylation. Since the use of doxorubicin is limited by toxicity, the combination could offer an effective treatment modality with lower toxicity for breast cancer.







Similar content being viewed by others
References
Appella E, Anderson CW (2001) Posttranslational modification and activation of p53 by genotoxic stresses. Eur J Biochem 268:2764–2772
Aviram A, Zimrah Y, Shaklai M, Nudelman A, Rephaeli A (1994) Comparison between the effect of BA and its prodrug pivaloyloxymethyl butyrate on histones hyperacetylation in an HL-60 leukemic cell line. Int J Cancer 56:906–909
Batova A, Shao L, Diccianni MB, Yu AL, Tanaka T, Rephaeli A, Nudelman A, Yu J (2002) The histone deacetylase inhibitor AN-9 has selective toxicity to acute leukemia and drug-resistant primary leukemia and cancer cell lines. Blood 100:3319–3324
Chen JS, Faller DV, Spanjaard RA (2003) Short-chain fatty acid inhibitors of histone deacetylases: promising anticancer therapeutics? Curr Cancer Drug Targets 3:219–236
Chou TC (1991) The median-effect principle and the combination index for quantitation of synergism and antagonism. In: Chou TC, Rideeout DC (eds) Synergism and antagonism in chemotherapy. Academic, New York, pp 66–102
Cutts SM, Rephaeli A, Nudelman A, Hmelnitsky I, Phillips DR (2001) Molecular basis for the synergistic interaction of adriamycin with the formaldehyde-releasing prodrug pivaloyloxymethyl butyrate (AN-9). Cancer Res 61:8194–8202
Cutts SM, Nudelman A, Pillay V, Spencer DMS, Levovich I, Rephaeli A, Phillips DR (2005) Formaldehyde-releasing prodrugs in combination with Adriamycin can overcome cellular drug resistance. Oncol Res 15:199–213
Davie JR (2003) Inhibition of histone deacetylase activity by butyrate. J Nutr 133:2485–2493
DeVita VT, Hellman S, Rosenberg SA (2001) Cancer: principles and practice of oncology, 6th edn. Williams and Wilkins, Lippincott
Emens LA, Davidson NE (2003) The follow-up of breast cancer. Semin Oncol 30:338–348
Gui CY, Ngo L, Xu WS, Richon VM, Marks PA (2004) Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. Proc Natl Acad Sci USA 101:1241–1246
Ju R, Muller MT (2003) Histone deacetylase inhibitors activate p21WAF1 expression via ATM. Cancer Res 63:2891–2897
Kalasz H (2003). Biological role of formaldehyde, and cycles related to methylation, demethylation, and formaldehyde production. Mini Rev Med Chem 3:175–192
Kasukabe T, Rephaeli A, Honma Y (1997) An anticancer derivative of BA (pivalyloxymethyl-butyrate) and daunorubicin cooperatively prolong survival of mice inoculated with monocytic leukemia cells. Br J Cancer 75:850–854
Kelly WK, Richon VM, O’Connor O, Curley T, MacGregor-Curtelli B, Tong W, Klang M, Schwartz L, Richardson S, Rosa E, Drobnjak M, Cordon-Cordo C, Chiao JH, Rifkind R, Marks PA Scher H (2003) Phase I clinical trial of histone deacetylase inhibitor: suberoylanilide hydroxamic acid administered intravenously. Clin Cancer Res 9:3578–3588
Kelly WK, O’Connor OA, Krug LM, Chiao JH, Heaney M, Curley T, Cortelli BM, Tong W, Secrist JP, Schwartz L, Richardson S, Chu E, Olgac S, Marks PA, Scher H, Richon VM (2005) Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol 23:3923–3931
Kim MS, Blake M, Baek JH, Kohlhagen G, Pommier Y, Carrier F (2003) Inhibition of histone deacetylase increases cytotoxicity to anticancer drugs targeting DNA. Cancer Res 63:7291–7300
Legube G, Trouche D (2003) Regulating histone acetyltransferases and deacetylases. EMBO Rep 4:944–947
Marks PA, Richon VM, Breslow R, Rifkind RA (2001) Histone deacetylase inhibitors as new cancer drugs. Curr Opin Oncol 13:477–483
McCaffrey TA, Agarwal LA, Weksler BB (1988) A rapid fluorometric DNA assay for the measurement of cell density and proliferation in vitro. In Vitro Cell Dev Biol 24:247–252
Melnick A, Licht JD (2002) Histone deacetylases as therapeutic targets in hematologic malignancies. Curr Opin Hematol 9:322–332
Nemunaitis JJ, Orr D, Eager R, Cunningham CC, Williams A, Mennel R, Grove W, Olson S (2003) Phase I study of oral CI-994 in combination with gemcitabine in treatment of patients with advanced cancer. Cancer J 9:58–66
Niitsu N, Kasukabe T, Yokoyama A, Okabe-Kado J, Yamamoto-Yamaguchi Y, Umeda M, Honma Y (2000) Anticancer derivative of butyric acid (pivalxymethyl butyrate) specifically potentiates the cytotoxicity of doxorubicin and danorubicin through the suppression of microsomal glycoside activity. Mol Pharmacol 58:27–36
Nudelman A, Gnizi E, Katz Y, Azulai R, Cohen-Ohana M, Zhuk R, Sampson SR, Langzam L, Fibach E, Prus E, Pugach V, Rephaeli A (2001) Prodrugs of butyric acid (III). Novel derivatives possessing increased aqueous solubility and potential for treating cancer and blood diseases. Eur J Med Chem 36:63–74
Nudelman A, Levovich I, Cutts SM, Phillips DR, Rephaeli A (2005) The role of intracellularly released formaldehyde and butyric acid, in the anticancer activity of acyloxyalkyl esters. J Med Chem 48:1042–1054
Patnaik A, Rowinsky EK, Villalona MA, Hammond LA, Britten CD, Siu LL, Goetz A, Felton SA, Burton S, Valone FH, Eckhardt SG (2002) A phase I study of pivaloyloxymethyl butyrate, a prodrug of the differentiating agent butyric acid, in patients with advanced solid malignancies. Clin Cancer Res 8:2142–2148
Prakash S, Foster BJ, Meyer M, Wozniak A, Heilbrun LK, Flaherty L, Zalupski M, Radulovic L, Valdivieso M, LoRusso PM (2001) Chronic oral administration of CI-994: a phase 1 study. Invest New Drugs 19:1–11
Reid T, Valone F, Lipera W, Irwin D, Paroly W, Natale R, Sreedharan S, Keer H, Lum B, Scappaticci F (2004) Phase II trial of the histone deacetylase inhibitor pivaloyloxymethyl butyrate (Pivanex, AN-9) in advanced non-small cell lung cancer. Lung Cancer 45:381–386
Rephaeli A, Rabizadeh E, Aviram A, Shaklai M, Ruse M, Nudelman A (1991) Derivatives of butyric acid as potential antineoplastic agents. Int J Cancer 49:66–72
Rephaeli A, Blank-Porat D, Tarasenko N, Entin-Meer M, Levovich I, Cutts SM, Phillips DR, Malik Z, Nudelman A (2005) In vivo and in vitro anti tumor activity of butyroyloxymethyl-diethyl phosphate (AN-7), a histone deacetylase inhibitor, on human prostate cancer. Int J Cancer 116:226–235
Rephaeli A, Entin-Meer M, Gruss-Fischer T, Tarasenko N, Bruachman I, Angel D, Haas-Kogan D, Nudelman A (2006). The selectivity and anti-metastatic activity of butyric acid prodrugs possessing oral bioavailability. Invest New Drugs (in press)
Rosato RR, Grant S (2003) Histone deacetylase inhibitors in cancer therapy. Cancer Biol Ther 2:30–37
Sandor V, Bakke S, Robey RW, Kang MH, Blagosklonny MV, Bender J, Brooks R, Piekarz RL, Tucker E, Figg WD, Chan KK, Goldspiel B, Fojo AT, Balcerzak SP, Bates SE (2002) Phase I trial of the histone deacetylase inhibitor, depsipeptide (FR901228, NSC 630176), in patients with refractory neoplasms. Clin Cancer Res 8:718–728
Taatjes DJ, Fenick DJ, Koch TH (1998) Epidoxoform: a hydrolytically more stable anthracycline–formaldehyde conjugate toxic to resistant tumor cells. J Med Chem 9(41):1306–1314
Tyihak BJ; Bocsi J; Timar F; Racz G; Szende B (2001) Formaldehyde promotes and inhibits the proliferation of cultured tumour and endothelial cells. Cell Prolif 34:135–141
Wolffe AP (1997) Transcriptional control. Sinful repression. Nature 387:16–17
Yu J, Zhang L (2005) The transcriptional targets of p53 in apoptosis control. Biochem Biophys Res Commun 31:851–858
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is supported by a grant 542/00-3 from Israel Science Foundation (A.R. and A.N.); a project grant from the Israel Cancer Research Fund, Israel Cancer Association; The Marcus Center for Pharmaceutical and Medicinal Chemistry at Bar Ilan University (A.N.), Australian Research Council (S.M.C. and D.R.P.). This work was performed by D. Engel as part of the requirements for Ph.D. degree in Bar Ilan University
Rights and permissions
About this article
Cite this article
Engel, D., Nudelman, A., Levovich, I. et al. Mode of interaction between butyroyloxymethyl-diethyl phosphate (AN-7) and doxorubicin in MCF-7 and resistant MCF-7/Dx cell lines. J Cancer Res Clin Oncol 132, 673–683 (2006). https://doi.org/10.1007/s00432-006-0116-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-006-0116-6