Abstract
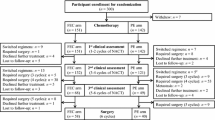
Purpose: To assess the efficacy and the toxicity of a new combination of epirubicin, cyclophosphamide and paclitaxel as neoadjuvant chemotherapy for breast cancer. Methods: Patients with stage II and III breast cancer received 3–4 cycles of epirubicin 75 mg/m2 plus cyclophosphamide 600 mg/m2 on day 1, and paclitaxel at a dose of 100 mg on days 1, 8, 15 and 22 on a 28-day cycle. Results: Forty-nine patients were enrolled in the study. Stage II was present in 16 patients (32.7%) and stage III in 33 patients (67.3%). Relevant toxicities were nausea/vomiting grades III–IV in 6 patients (12.2%) and neutropenia grade III–IV in 33 patients (67.3%). The overall clinical response rate was 83.7%. Partial response was observed in 25 patients (51%), complete response in 16 patients (32.7%), stable disease in 7 patients (14.3%) and progression in 1 patient. Thirty-three non-inflammatory breast cancer patients underwent surgery, 29 with breast-conserving surgery (87.9%). Pathological complete response was found in 5 patients (15.1%). Conclusions: The combination of epirubicin, cyclophosphamide and weekly paclitaxel as given in this study is safe and shows good activity in the neoadjuvant setting of stage II and III breast cancer patients.
Similar content being viewed by others
References
Bear HD, Anderson S, Brown A et al (2003) The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project protocol B-27. J Clin Oncol 21:4165–4174
Bonadonna G, Valagussa P, Brambilla C et al (1998) Primary chemotherapy in operable breast cancer: eight-year experience at the Milan Cancer Institute. J Clin Oncol 16:93–100
Buzdar AU, Singletary SE, Theriault RL et al (1999) Prospective evaluation of paclitaxel versus combination chemotherapy with fluorouracil, doxorubicin, and cyclophosphamide as neoadjuvant therapy in patients with operable breast cancer. J Clin Oncol 17:3412–3417
Buzdar AU, Ibrahim NK, Francis D et al (2005) Significantly higher pathologic complete remission rate after neoadjuvant with trastuzumab, pacltaxel, and epirubicin chemotherapy: Results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol 23:3676–3685
Chevallier B, Roche H, Olivier JP et al (1993) Inflammatory breast cancer. Pilot study of intensive induction chemotherapy (FEC-HD) results in a high histologic response rate. Am J Clin Oncol 16:223–228
Chollet P, Amat S, Cure H et al (2002) Prognostic significance of a complete pathological response after induction chemotherapy in operable breast cancer. Br J Cancer 86:1041–1046
Cure H, Amat S, Penault-Llorca F et al (2002) Prognostic value of residual node involvement in operable breast cancer after induction chemotherapy. Breast Cancer Res Treat 76(Suppl 1):37–45
Dieras V, Fumoleau P, Romieu G et al (2004) Randomized parallel study of doxorubicin plus paclitaxel and doxorubicin plus cyclophosphamide as neoadjuvant treatment of patients with breast cancer. J Clin Oncol 22:4958–4965
Evans TRJ, Gould A, Foster E et al (2002) Phase III randomized trial of adriamycin and docetaxel versus adriamycin and cyclophosphamide as primary medical therapy in women with breast cancer. Proc Am Soc Clin Oncol 21 (Abstr 35)
Ferriere JP, Assier I, Cure H et al (1998) Primary chemotherapy in breast cancer: correlation between tumor response and patient outcome. Am J Clin Oncol 21:117–120
Fisher B, Bryant J, Wolmark N et al (1998) Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 16:2672–2685
Gianni L, Baselga J, Eiermann W et al (2002) First report of the European Cooperative Trial in operable breast cancer (ECTO): effects of primary systemic therapy (PST) on local-regional disease. Proc Am Soc Clin Oncol 21 (Abstr 132)
Green MC, Buzdar AU, Smith T et al (2005) Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol 23:5983–5992
Honkoop AH, van Diest PJ, de Jong JS et al (1998) Prognostic role of clinical, pathological and biological characteristics in patients with locally advanced breast cancer. Br J Cancer 77:621–626
Hutcheon AW, Heys SD, Sarkar TK et al (2003) Docetaxel primary chemotherapy in breast cancer: a five-year update of the Aberdeen trial. Breast Cancer Res Treat 82(Suppl 1) (Abstr 11)
Jakesz R for the ABCSG (2001) Comparasion of pre-vs. postoperative chemotherapy in breast cancer patients: four-year results of Austrian Breast, Colorectal Cancer Study Group (ABCSG) trial 7. Proc Am Soc Clin Oncol 20 (Abstr 125)
Kuerer HM, Newman LA, Smith T et al (1999) Clinical course of cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol 17:460–469
Lau DH, Xue L, Young LJ et al (1999) Paclitaxel (Taxol): an inhibitor of angiogenesis in a highly vascularized transgenic breast cancer. Cancer Biother Radiopharm 14:31–36
Luporsi L, Vanlemmens L, Coudert B et al (2000) 6 cycles of FEC 100 vs 6 cycles of epirubicin-docetaxel as neoadjuvant chemotherapy in operable breast cancer patients: preliminary results of a randomized phase II trial of Girec S01. Proc Am Soc Clin Oncol 19 (Abstr 355)
Machiavelli MR, Romero AO, Perez JE et al (1998) Prognostic significance of pathological response of primary tumor and metastatic axillary lymph nodes after neoadjuvant chemotherapy for locally advanced breast carcinoma. Cancer J Sci Am 4:125–131
MauriacL, MacGrogan G, Avril A et al (1999) Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: a unicentre randomized trial with a 124-month median follow-up. Institut Bergonie Bordeaux Groupe Sein (IBBGS). Ann Oncol 10:47–52
Sataloff DM, Mason BA, Prestipino AJ et al (1995) Pathologic response to induction chemotherapy in locally advanced carcinoma of the breast: a determinant of outcome. J Am Coll Surg 180:297–306
Seidman AD, Berry D, Cirrincione C et al (2004) CALGB 9840: Phase III study of weekly (W) paclitaxel (P) via 1-hour(h) infusión versus standard infusión (S) 3 h infusión every third week in the treatment of metastatic breast cancer (MBC), with trastuzumab (T) for HER2 positive MBC and randomized for T in HER2 normal MBC. J Clin Oncol In: ASCO annual meeting proceedings (post-meeting edition) 22 (Abstr 512)
Semiglazov VF, Bojok AA, Arsumanov AS et al (2002) Breast conserving surgery after neoadjuvant chemotherapy paclitaxel + doxorubicin vs fluorouracil + doxorubicin + cyclophosphamide. Breast Cancer Res Treat 76(Suppl 1) (Abstr 159)
Singletary SE, Allred C, Ashley P et al (2002) Revision of the American Joint Committee on Cancer Staging System for Breast Cancer. J Clin Oncol 20:3628–3636
Smith IA, Miller ID (2001) Issues involved in research into the neoadjuvant treatment of breast cancer. Anticancer Drugs 12(Suppl 1):S25–S29
Smith IC, Heys SD, Hutcheon AW et al (2002) Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J Clin Oncol 20:1456–1466
Valero V, Buzdar AU, Kau S-W et al (2002) Pathologically involved axillary nodes following primary chemotherapy in locally advanced breast cancer is an early biological prognostic factor for long-term outcome in patients with LABC. Ann Oncol 13(Suppl 5) (Abstr 132P)
van der Hage JA, van de Velde CJ, Julien JP et al (2001) Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol 19:4224–4237
Vinholes J, Bouzid K, Salas F et al (2001) Preliminary results of a multicentre phase III trial of Taxotere and doxorubicin versus 5-fluorouracil, doxorubicin and cyclophosphamide in patients with unresectable locally advanced breast cancer. In: Proceedings of the American Society on Clinical Oncology 20 (Abstr 101)
Wang LG, Liu XM, Kreis W et al (1999) The effect of antimicrotubule agents on signal transduction pathways of apoptosis: a review. Cancer Chemother Pharmacol 44:355–361
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aguiar Bujanda, D., Bohn Sarmiento, U., Cabrera Suárez, M.Á. et al. Epirubicin, cyclophosphamide and weekly paclitaxel as neoadjuvant chemotherapy for stage II and III breast cancer. J Cancer Res Clin Oncol 132, 332–338 (2006). https://doi.org/10.1007/s00432-006-0079-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-006-0079-7