Abstract
Purpose
To test the hypothesis that radiation-induced, transient G2/M arrest could potentially sensitize tumor cells to a subsequent, well-timed radiation dose.
Methods
PC-3 human prostate cancer cells were treated using either radiotherapy or 186Re-labeled hydroxyethylidene diphosphonate (186Re-HEDP) treatment in different combinations. The resulting cell cycle shift and clonogenic cell death were analyzed by DNA flow cytometry and colony forming cell assay, respectively.
Results
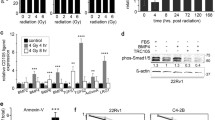
Radiation doses of 4 Gy and 8 Gy induced a transient G2/M arrest, with a maximum after approximately 16 h. The presence of 2 mM pentoxifylline effectively abrogated this radiation-induced G2 M arrest, confirming a cell-cycle checkpoint-mediated effect. A second dose of 4 Gy, timed at the height of the G2/M arrest, significantly increased clonogenic cell-kill compared to delivery after a suboptimal interval (10 h, 20 h or 25 h after the first radiation fraction). Moreover, timed second doses of 2 Gy, 3 Gy or 4 Gy yielded improved normalized treatment effects compared to non-pretreated control. Radionuclide treatment of PC-3 cells, using 186Re-HEDP (0.74 MBq/ml and 1.48 MBq/ml; total dose: 4.1 and 8.2 Gy, respectively) also induced a dose-dependent G2/M accumulation, which sensitized the cells to a subsequent external radiation dose of 2 Gy or 4 Gy. The observed pattern of cell-cycle shift towards a predominance of the G2/M phase is in line with the lack of functional p53 in this cell line.
Conclusions
Radiation-induced cell-cycle shift was shown to effectively confer increased radiosensitivity to prostate tumor cells. Optimally timed combination of radiotherapy and radionuclide therapy could thus significantly increase treatment efficacy.






Similar content being viewed by others
References
Bedford JS, Mitchell JB, Fox M (1980) Variations in responses of several mammalian cell lines to low dose-rate irradiation. In: Meyn RE, Withers HR (eds) Radiation biology in cancer research. Raven, New York, pp 241–262
Blagosklonny MV, Pardee AB (2001) Exploiting cancer cell cycling for selective protection of normal cells. Cancer Res 61:4301–4305
Bohm (2000) Influence of pentoxyfilline, A-802710, propentofylline and A-802715 (Hoechst) on the expression of cell cycle blocks and S-phase content after irradiation damage. Biochim Biophys Acta 1499:1–10
Deweese TL, Shipman JM, Dillehay LE, Nelson WG (1998) Sensitivity of human prostatic carcinoma cell lines to low-dose rate radiation exposure. J Urol 159:591–598
Elledge SJ (1996) Cell cycle checkpoints: preventing an identity crisis. Science 274:1664–1672
Formenti SC, Symmans WF, Volm M, Skinner K, Cohen D, Spicer D, Danenberg PV (1999) Concurrent paclitaxel and radiationtherapy for breast cancer. Sem Rad Oncol 9:34–42
Fowler JF (1990) Radiobiological aspects of low-dose rate in radioimmunotherapy. Int J Rad Oncol Biol Phys 18:1261–1269
Geldof AA, Rooij L de, Versteegh RT, Newling DWW, Teule GJJ (1999a) Combination 186Re-HEDP and cisplatin supra-additive treatment effects in prostate cancer cells. J Nucl Med 40:667–671
Geldof AA, Kruit A, Newling DWW, Slotman BJ (1999b) Synergism between cisplatin and radiotherapy in an in vitro prostate tumor cell line. Anticancer Res 19:505–508
Griffith TD, Tolmach LJ (1976) Lethal response of HeLa cells to X-irradiation in the latter part of the generation cycle. Biophys J 16:303–318
Gupta N, Hu LJ, Deen DF (1997) Cytotoxicity and cell-cycle effects of paclitaxel when used as a single agent and in combination with ionizing radiation. Int J Rad Oncol Biol Phys 37:885–895
Hall EJ (1994) Radiosensitivity and cell age in the mitotic cycle. In: Hall EJ (ed) Radiobiology for the radiologist, 4th ed. Lippincott, Philadelphia, pp 91–105
Hartwell LH, Kastan MB (1994) Cell cycle control and cancer. Science 226:1821–1828
Hennequin C, Giocanti N, Favaudon V (1996) Interaction of ionizing radiation with paclitaxel (Taxol) and docetaxel (Taxotere) in HeLa and SQ20Bcells. Cancer Res 56:1842–1850
Innocente SA, Abrahamson JLA, Cogswell JP, Lee JM (1999) p53 regulates a G2 checkpoint through cyclin B1. Proc Natl Acad Sci USA 96:2147–2152
Isaacs WB, Carter BS, Ewing CM (1991) Wild-type p53 suppresses growth of human prostate cancer cells containing mutant p53 alleles. Cancer Res 51:4716–4720
Kal HB, Barendsen GW, Bakker-van Haue R, Roeke H (1975) Increased radiosensitivity of rat rhabdomyosarcoma cells induced by protracted irradiation. Rad Res 63:521–530
Kano Y, Akutsu M, Tsunoda S, Furata M, Yazawa Y, Ando J (1998) Schedule-dependent synergism and antagonism between paclitaxel and methotrexate in human carcinoma cell lines. Oncol Res 10:347–354
Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW (1991) Participation of p53 protein in the cellular response to DNA damage. Cancer Res 51:6304–6311
Koutcher JA, Motwani M, Zakian KL, Li XK, Matei C, Dyke JP, Ballon D, Yoo HH, Schwartz GK (2000) The in vivo effect of Bryostatin-1 on paclitaxel-induced tumor growth, mitotic entry and blood flow. Clin Cancer Res 6:1498–1507
Kuerbitz SJ, Plunkett BS, Walsh WV, Kastan MB (1992) Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci USA 89: 7491–7495
Lundberg AS, Weinberg RA (1999) Control of the cell cycle and apoptosis. Europ J Cancer 35:531–539
Mastbergen SC, Duivenvoorden I, Versteegh RT, Geldof AA (2000) Cell cycle arrest and clonogenic tumor cell kill by divergent chemotherapeutic drugs. Anticancer Res 20:1833–1838
Mitchell JB, Bedford JS, Bailey SM (1979) Dose-rate effects in mammalian cells in culture III. Comparison of cell killing and cell proliferation during continuous irradiation for six different cell lines. Rad Res 79:537–551
Russell KJ, Wiens LW, Demers GW, Galloway DA, Le T, Rice GC, Bianco JA, Singer JW, Groudine M (1996) Preferential radiosensitization of G1 checkpoint-deficient cells by methylxanthines. Int J Rad Oncol Biol Phys 36:1099–1106
Schiff PB, Fant J, Horowitz SB (1979) Promotion of microtubule assembly in vitro by taxol. Nature 22:665–667
Schiff PB, Horowitz SB (1980) Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci USA 77:1561–1565
Sherr CJ (1996) Cancer cell cycles. Science 274:1672–1677
Sinclair WK (1968) Cyclic X-ray responses in mammalian cells in vitro. Rad Res 33:620–643
Van Buul PPW, van Duyn-Goedhart A, Beumers T, Bootsma AL (2001) Role of cell cycle control in radiosensitization of mouse spermatogonial stem cells. Int J Radiat Biol 77:357–363
Van Leeuwen-Stok EA, Jonkhoff AR, Visser-Platier AW, Drager LM, Teule GJ, Huijgens PC, Schuurhuis GJ (1998) Cell cycle dependency of 67gallium uptake and cytotoxicity in human cell lines of hematological malignancies. Leuk Lymph 31:533–544
Wang S, Guo CY, Castillo A, Dent P, Grant S (1998) Effect of Bryostatin 1 on taxol-induced apoptosis and cytotoxicity in human leukemia cells (U937). Biochem Pharmacol 56:635–644
Wang XW, Zhan Q, Coursen JD, Khan MA, Ktny U, Yu L, Hollander MC, O'connor PM, Fornace AJ, Harris CC (1999) GADD45 induction of a G2/M cell cycle checkpoint. Proc Natl Acad Sci USA 96:3706–3711
Zoli W, Ricotto L, Barzanti F, Dal Susino M, Frassineti GL, Miland C, Casadei Giunchi D, Amadori D (1999) Schedule-dependent interaction of doxorubicin, paclitaxel and gemcitabine in human breast cancer cell lines. Int J Cancer 80: 413–416
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Geldof, A.A., Plaizier, M.A., Duivenvoorden, I. et al. Cell cycle perturbations and radiosensitization effects in a human prostate cancer cell line. J Cancer Res Clin Oncol 129, 175–182 (2003). https://doi.org/10.1007/s00432-002-0412-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-002-0412-8