Abstract
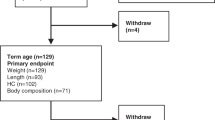
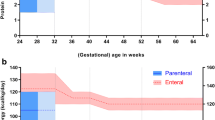
To evaluate the influence of early nutritional intake on the growth pattern of very preterm infants. This was an observational study including 109 newborns (< 32 weeks gestational age). Perinatal morbidities, nutritional therapy (first four weeks of life), and weight, length, and head circumference (HC) growth at term-equivalent age were evaluated. Growth restriction was defined as a difference > 1.2 SD between the birth and term age measurements. Growth restriction at term-equivalent age: 52.3% (weight), 42.9% (length), and 22% (HC). Morbidities were positively correlated with nutrition therapy and negatively correlated with the total energy provision: protein ratio. The duration of parenteral nutrition, the time to reach full enteral feedings, and the total energy provision: protein ratio were significantly correlated. Nutrient intake influenced weight, length, and HC growth, and cumulative energy deficit was significantly associated with HC growth restriction.
Conclusion: Perinatal morbidities interfere with nutritional therapy and early nutrient intake, leading to insufficient energy and energy provision: protein ratio for growth.
What is Known: • The intake of macronutrients early in life, mainly protein, is important for the optimal growth of pretem infants. • The severity of morbidities and low gestational ages impact the nutritional management of preterm infants. | |
What is New: • The number of morbidities, reflecting the severity of the neonatal clinical course, had a detrimental effect on the nutritional therapy and nutrients intake. • The inadequate energy provision per gram of protein ratio was significantly associated with growth restriction in all growth measures at the second week of life, persisting for head circumference up to the fourth week, highlighting the importance of its measurement, as it could be a precocious sign of development risk. |

Similar content being viewed by others
Data availability
The data presented in this study are available upon request from the corresponding author.
Abbreviations
- BPD:
-
Bronchopulmonary dysplasia
- ESPGHAN:
-
The European Society of Pediatric Gastroenterology, Hepatology and Nutrition
- EUGR:
-
Extrauterine growth restriction
- GA:
-
Gestational age
- HC:
-
Head circumference
- IFF/FIOCRUZ:
-
Instituto Fernandes Figueira
- IMV:
-
Invasive mechanical ventilation
- IVH:
-
Intraventricular hemorrhage
- NB:
-
Newborns
- NEC:
-
Necrotizing enterocolitis
- NICU:
-
Neonatal intensive care unit
- PMA:
-
Postmenstrual age
- PN:
-
Parenteral nutrition
- ROP:
-
Retinopathy of prematurity
- SGA:
-
Small for gestational age
References
Horbar JD, Ehrenkranz RA, Badger GJ, Edwards EM, Morrow KA, Soll RF et al (2015) Weight growth velocity and postnatal growth failure in infants 501 to 1500 Grams: 2000–2013. Pediatrics 136:e84–e92
Ong KK, Kennedy K, Castañeda-Gutiérrez E, Forsyth S, Godfrey KM, Koletzko B et al (2015) Postnatal growth in preterm infants and later health outcomes: a systematic review. Acta Paediatr 104:974–986
Fenton TR, Cormack B, Goldberg D, Nasser R, Alshaikh B, Eliasziw M et al (2020) Extrauterine growth restriction and postnatal growth failure are misnomers for preterm infants. J Perinatol 40:704–714
Renau MI, Aldecoa-Bilbao V, Esponera CB, de Mendoza BRH, Sanz MI, Iglesias-Platas I (2019) Applying methods for postnatal growth assessment in the clinical setting: evaluation in a longitudinal cohort of very preterm infants. Nutrients 11:2772
Reddy KV, Sharma D, Vardhelli V, Bashir T, Deshbotla SK, Murki S (2019) Comparison of fenton 2013 growth curves and intergrowth-21 growth standards to assess the incidence of intrauterine growth restriction and extrauterine growth restriction in preterm neonates ≤ 32 weeks. J Matern Fetal Neonatal Med 34(16):2634–2641
Maiocco G, Migliaretti G, Cresi F, Peila C, Deantoni S, Trapani B et al (2020) Evaluation of extrauterine head growth from 14–21 days to discharge with longitudinal intergrowth-21st charts: a new approach to identify very preterm infants at risk of long-term neurodevelopmental impairment. Front Pediatr 8:572930
Tuzun F, Yucesoy E, Baysal B, Kumral A, Duman N, Ozkan H (2018) Comparison of INTERGROWTH-21 and fenton growth standards to assess size at birth and extrauterine growth in very preterm infants. J Matern Fetal Neonatal Med 31:2252–2257
González-García L, García-López E, Fernández-Colomer B, Mantecón-Fernández L, Lareu-Vidal S, Suárez-Rodríguez M et al (2021) Extrauterine growth restriction in very low birth weight infants: concordance between fenton 2013 and INTERGROWTH-21st growth charts. Front Pediatr 9:690788
Viswanathan S, Osborn E, Jadcherla S (2022) Predictive ability of postnatal growth failure for adverse feeding-related outcomes in preterm infants: an exploratory study comparing Fenton with INTERGROWTH-21st preterm growth charts. J Matern Fetal Neonatal Med 35:5470–5477
Zozaya C, Díaz C (2018) Saenz De Pipaón M. How should we define postnatal growth restriction in preterm infants? Neonatology 114:177–180
Rochow N, Raja P, Liu K, Fenton T, Landau-Crangle E, Göttler S et al (2016) Physiological adjustment to postnatal growth trajectories in healthy preterm infants. Pediatr Res 79:870–879
Embleton ND, Moltu SJ, Lapillonne A, van den Akker CHP, Carnielli V, Fusch C et al (2023) Enteral nutrition in preterm infants (2022): a position paper from the ESPGHAN committee on nutrition and invited experts. JPGN 76:248–268
Embleton ND, Cleminson J, Zalewski S (2017) What growth should we aim for in preterm neonates? PCH 27:18–22
Papile LA, Burstein J, Burstein R, Koffler H (1978) Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 Gm. J Pediatr 92:529–534
Bancalari E, Jain D (2019) Bronchopulmonary dysplasia: 50 years after the original description. Neonatology 115:384–391
Chiang MF, Quinn GE, Fielder AR, Ostmo SR, Chan PRV, Berrocal A et al (2021) International classification of retinopathy of prematurity, 3rd ed. Ophthalmology 128:e51–e68
Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L et al (1978) Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg 187(1):1–7
Milanesi BG, Lima PAT, Villela LD, Martins AS, Gomes-Junior SCS, Moreira MEL, Meio MDBB (2021) Assessment of early nutrition intake in preterm infants with bronchopulmonary dysplasia: a cohort study. Eur J Pediatr 180(5):1423–1430
Fenton TR, Kim JH (2013) A systematic review and meta-analysis to revise the fenton growth chart for preterm infants. BMC Pediatr 13:59
Goldberg DL, Becker PJ, Brigham K, Carlson S, Fleck L, Gollins L et al (2018) Identifying malnutrition in Preterm and neonatal populations: recommended indicators. J Acad Nutr Diet 118:1571–1582
Zin OA, Soares FVM, Abranches AD, Costa ACC, Villela LD, Moreira MEL (2019) Analysis of the differences between the prescribed and the administered diet to preterm infants using an electronic tool. Rev Paul Pediatr 37:472–478
Embleton ND, van den Akker CHP (2019) Protein intakes to optimize outcomes for preterm infants. Semin Perinatol 43:151154
Yitayew M, Chahin N, Rustom S, Thacker LR, Hendricks-Muñoz KD (2021) Fenton vs. intergrowth-21st: postnatal growth assessment and prediction of neurodevelopment in preterm infants. Nutrients 13:2841
Villar J, Giuliani F, Bhutta ZA, Bertino E, Ohuma EO, Ismail, et al (2015) Postnatal growth standards for preterm infants: the preterm postnatal follow-up study of the INTERGROWTH-21st project. Lancet Glob Health 3:e681–e691
Lygerou I, Ilia S, Briassoulis P, Manousaki A, Koropouli M, Hatzidaki E, Briassoulis G (2023) The impact of estimated energy and protein balances on extrauterine growth in preterm infants. Nutrients 15:3556. https://doi.org/10.3390/nu15163556
Moltu SJ, Bronsky J, Embleton N, Gerasimidis K, Indrio F, Köglmeier J et al (2021) Nutritional management of the critically ill neonate: a position paper of the ESPGHAN committee on nutrition. JPGN 73:274–289
Fivez T, Kerklaan D, Mesotten D, Verbruggen S, Wouters PJ, Vanhorebeek I et al (2016) Early versus late parenteral nutrition in critically ill children. N Engl J Med 374:1111–1122
Raghuram K, Yang J, Church PT, Cieslak Z, Synnes A, Mukerji A et al (2017) Head growth trajectory and neurodevelopmental outcomes in preterm neonates. Pediatrics 140:e20170216
Wang S, Fan P, Xiong D, Yang P, Zheng J, Zhao D (2018) Assessment of neonatal brain volume and growth at different postmenstrual ages by conventional MRI. Medicine 97:e11633
Binder C, Buchmayer J, Thajer A, Giordano V, Schmidbauer V, Harreiter K et al (2021) Association between fat-free mass and brain size in extremely preterm infants. Nutrients 13:4205
Funding
Partial financial support was received from Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro – FAPERJ – Grant number E-26/201.116/2021.
Author information
Authors and Affiliations
Contributions
Méio MDBB contributed to the conception of the study and was responsible for the coordination of the study, contributing to the analysis and interpretation of the data, the writing of the manuscript and its critical revision. Salgado GGM and Villela LD contributed to the analysis and interpretation of the data, the writing of the manuscript and its critical revision. Salgado GGM, Villela LD, Lima PAT, and Milanesi BG contributed to the material preparation and data collection. da Costa ACC performed the statistical analysis and contributed to the interpretation of the results and the writing of the manuscript. Moreira MEL contributed to the acquisition of financial resources for the study, the interpretation of the results and the critical revision of the manuscript. All the authors have read and approved the final manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Informed consent
Informed consent was obtained from all subjects involved in the study. This study was approved by the Ethics Committee of Instituto Fernandes Figueira (IFF/FIOCRUZ) on December 11th, 2015, under protocol code number CAAEE 50243615.0.0000.5269, in accordance with the Declaration of Helsinki.
Competing interests
The authors declare no competing interests.
Disclosure
All the authors state that artificial intelligence was not used during the entire process of interpreting the data, discussing the results, or writing the manuscript.
Additional information
Communicated by Daniele De Luca
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Méio, M.D.B.B., de Miranda Salgado, G.G., Villela, L.D. et al. Influence of morbidity, early nutritional intake, and total energy: protein ratio on longitudinal extrauterine growth restriction of very preterm newborns at term-equivalent age: an observational study. Eur J Pediatr (2024). https://doi.org/10.1007/s00431-024-05595-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00431-024-05595-3