Abstract
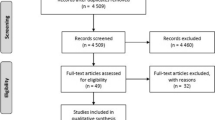
This systematic review and meta-analysis aimed to evaluate the effectiveness of COVID-19 vaccines among children and adolescents against SARS-CoV-2 variants. We searched PubMed, Embase, Web of Science, the Cochrane Library, and ClinicalTrials.gov for studies published on or before June 20, 2023. Studies evaluating the effectiveness of COVID-19 vaccines in children and adolescents (≤ 18 years of age) were included. Data extraction, quality assessment, and analysis were conducted following PRISMA guidelines. Ten studies were included, comprising five cohort studies (527,778 participants) and four case-control studies (1,477,422 participants). The overall vaccine effectiveness (VE) against SARS-CoV-2 variants was 68% (95% CI = 60–74%). In terms of age, the VE was higher in adolescents aged 12–18 years [69%(95% CI = 61–75%)] than in children aged 5–11 years [44%(95% CI = 1–68%)]. “Fully vaccinated” may offer greater protection than “partially vaccinated,” with a VE of 71% (95%CI = 59–79%) and 66% (95%CI = 51–76%), respectively.
Conclusion: This meta-analysis presents moderate-quality evidence that the COVID-19 vaccine is effective in safeguarding children and adolescents from the SARS-CoV-2 variant. Being fully vaccinated may offer greater protection than being partially vaccinated. Nevertheless, additional high-quality controlled trials are required to verify this finding.
What is Known: • The COVID-19 pandemic has led to the rapid development and deployment of vaccines worldwide. Children and adolescents are a unique population for vaccination, and the effectiveness of vaccines against SARS-CoV-2 variants in this age group is of concern. | |
What is New: • The COVID-19 vaccine is effective in protecting children and adolescents against the SARS-CoV-2 variant. Being fully vaccinated may offer greater protection than being partially vaccinated. |



Similar content being viewed by others
Data availability
The data analyzed in this study is available from the corresponding author on reasonable request.
References
Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK et al (2021) Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet 397(10277):881–891. https://doi.org/10.1016/S0140-6736(21)00432-3
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R et al (2021) Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384(5):403–416. https://doi.org/10.1056/NEJMoa2035389
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S et al (2020) Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 383(27):2603–2615. https://doi.org/10.1056/NEJMoa2034577
Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS et al (2021) Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 397(10275):671–681. https://doi.org/10.1016/S0140-6736(21)00234-8
Hall VJ, Foulkes S, Saei A, Andrews N, Oguti B, Charlett A et al (2021) COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet 397(10286):1725–1735. https://doi.org/10.1016/S0140-6736(21)00790-X
Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA et al (2021) BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 384(15):1412–1423. https://doi.org/10.1056/NEJMoa2101765
Thompson MG, Burgess JL, Naleway AL, Tyner HL, Yoon SK, Meece J et al (2021) Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers - eight U.S. locations, December 2020-March 2021. MMWR Morb Mortal Wkly Rep 70(13):495–500. https://doi.org/10.15585/mmwr.mm7013e3
Gao P, Kang LY, Liu J, Liu M (2023) Immunogenicity, effectiveness, and safety of COVID-19 vaccines among children and adolescents aged 2–18 years: an updated systematic review and meta-analysis. World J Pediatr 1–14. https://doi.org/10.1007/s12519-022-00680-9
Denina M, Pruccoli G, Scolfaro C, Mignone F, Zoppo M, Giraudo I et al (2020) Sequelae of COVID-19 in hospitalized children: a 4-months follow-up. Pediatr Infect Dis J 39(12):e458–e459. https://doi.org/10.1097/INF.0000000000002937
Bottino I, Patria MF, Milani GP, Agostoni C, Marchisio P, Lelii M et al (2021) Can asymptomatic or non-severe SARS-CoV-2 infection cause medium-term pulmonary sequelae in children. Front Pediatr 9:621019. https://doi.org/10.3389/fped.2021.621019
Cao Y, Wang J, Jian F, Xiao T, Song W, Yisimayi A et al (2022) Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 602(7898):657–663. https://doi.org/10.1038/s41586-021-04385-3
García JE, González-López VA, Tasca GH (2022) Multiple partition Markov model for B.1.1.7, B.1.351, B.1.617.2, and P.1 variants of SARS-CoV 2 virus. Comput Stat :1–37. https://doi.org/10.1007/s00180-022-01291-8
Garcia-Beltran WF, Lam EC, St Denis K, Nitido AD, Garcia ZH, Hauser BM et al (2021) Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 184(9):2372-2383.e9. https://doi.org/10.1016/j.cell.2021.03.013
Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM et al (2021) Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 596(7871):276–280. https://doi.org/10.1038/s41586-021-03777-9
Wang CS, Doma R, Westbrook AL, Johnson J, Anderson EJ, Greenbaum LA et al (2023) Vaccine attitudes and COVID-19 vaccine intention among parents of children with kidney disease or primary hypertension. Am J Kidney Dis 81(1):25-35.e1. https://doi.org/10.1053/j.ajkd.2022.04.011
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Sterne J, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898. https://doi.org/10.1136/bmj.l4898
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919. https://doi.org/10.1136/bmj.i4919
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
Fleming-Dutra KE, Britton A, Shang N, Derado G, Link-Gelles R, Accorsi EK et al (2022) Association of prior BNT162b2 COVID-19 vaccination with symptomatic SARS-CoV-2 infection in children and adolescents during omicron predominance. JAMA 327(22):2210–2219. https://doi.org/10.1001/jama.2022.7493
Florentino P, Alves F, Cerqueira-Silva T, Oliveira VA, Júnior J, Jantsch AG et al (2022) Vaccine effectiveness of CoronaVac against COVID-19 among children in Brazil during the Omicron period. Nat Commun 13(1):4756. https://doi.org/10.1038/s41467-022-32524-5
Tartof SY, Frankland TB, Slezak JM, Puzniak L, Hong V, Xie F et al (2022) Effectiveness associated with BNT162b2 vaccine against emergency department and urgent care encounters for Delta and Omicron SARS-CoV-2 infection among adolescents aged 12 to 17 years. JAMA Netw Open 5(8):e2225162. https://doi.org/10.1001/jamanetworkopen.2022.25162
Oliveira CR, Niccolai LM, Sheikha H, Elmansy L, Kalinich CC, Grubaugh ND et al (2022) Assessment of clinical effectiveness of BNT162b2 COVID-19 vaccine in US adolescents. JAMA Netw Open 5(3):e220935. https://doi.org/10.1001/jamanetworkopen.2022.0935
Ng OT, Koh V, Chiew CJ, Marimuthu K, Thevasagayam NM, Mak TM et al (2022) Impact of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination and pediatric age on Delta variant household transmission. Clin Infect Dis 75(1):e35–e43. https://doi.org/10.1093/cid/ciac219
Ionescu IG, Skowronski DM, Sauvageau C, Chuang E, Ouakki M, Kim S et al (2023) BNT162b2 effectiveness against Delta and Omicron variants of SARS-CoV-2 in adolescents aged 12–17 years, by dosing interval and duration. J Infect Dis. https://doi.org/10.1093/infdis/jiad006
Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON et al (2021) Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet 398(10309):1407–1416. https://doi.org/10.1016/S0140-6736(21)02183-8
Reis BY, Barda N, Leshchinsky M, Kepten E, Hernán MA, Lipsitch M et al (2021) Effectiveness of BNT162b2 vaccine against Delta variant in adolescents. N Engl J Med 385(22):2101–2103. https://doi.org/10.1056/NEJMc2114290
Lutrick K, Rivers P, Yoo YM, Grant L, Hollister J, Jovel K et al (2021) Interim estimate of vaccine effectiveness of BNT162b2 (Pfizer-BioNTech) vaccine in preventing SARS-CoV-2 infection among adolescents aged 12–17 years - Arizona, July-December 2021. MMWR Morb Mortal Wkly Rep 70(5152):1761–1765. https://doi.org/10.15585/mmwr.mm705152a2
Powell AA, Kirsebom F, Stowe J, Ramsay ME, Lopez-Bernal J, Andrews N (2023) Protection against symptomatic infection with delta (B.1.617.2) and omicron (B.1.1.529) BA.1 and BA.2 SARS-CoV-2 variants after previous infection and vaccination in adolescents in England, August 2021-March, et al 2022 a national, observational, test-negative, case-control study. Lancet Infect Dis 23(4):435–444. https://doi.org/10.1016/S1473-3099(22)00729-0
Dunkle LM, Kotloff KL, Gay CL, Áñez G, Adelglass JM, Barrat Hernández AQ et al (2022) Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N Engl J Med 386(6):531–543. https://doi.org/10.1056/NEJMoa2116185
Smith LE, Amlôt R, Weinman J, Yiend J, Rubin GJ (2017) A systematic review of factors affecting vaccine uptake in young children. Vaccine 35(45):6059–6069. https://doi.org/10.1016/j.vaccine.2017.09.046
Galanis P, Vraka I, Siskou O, Konstantakopoulou O, Katsiroumpa A, Kaitelidou D (2022) Willingness, refusal and influential factors of parents to vaccinate their children against the COVID-19: a systematic review and meta-analysis. Prev Med 157:106994. https://doi.org/10.1016/j.ypmed.2022.106994
Wiysonge CS, Ndwandwe D, Ryan J, Jaca A, Batouré O, Anya BM et al (2022) Vaccine hesitancy in the era of COVID-19: could lessons from the past help in divining the future. Hum Vaccin Immunother 18(1):1–3. https://doi.org/10.1080/21645515.2021.1893062
Wang K, Wong EL, Cheung AW, Chung VC, Wong CH, Dong D et al (2022) Impact of information framing and vaccination characteristics on parental COVID-19 vaccine acceptance for children: a discrete choice experiment. Eur J Pediatr 181(11):3839–3849. https://doi.org/10.1007/s00431-022-04586-6
Liu E, Smyth RL, Li Q, Qaseem A, Florez ID, Mathew JL et al (2022) Guidelines for the prevention and management of children and adolescents with COVID-19. Eur J Pediatr 181(12):4019–4037. https://doi.org/10.1007/s00431-022-04615-4
Zeng B, Gao L, Zhou Q, Yu K, Sun F (2022) Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern: a systematic review and meta-analysis. BMC Med 20(1):200. https://doi.org/10.1186/s12916-022-02397-y
Acknowledgements
Thanks to all authors for their contributions to this article.
Funding
This study was supported by the National Natural Science Foundation of China (No. U22A20285), the National Natural Science Foundation of China (No. 82160433), the Key R&D Project of Autonomous Region (No. 2023BEG02018), the Ningxia Medical University General Hospital “Medical Engineering Special” (No. NYZYYG-001), the Scientific Research Project of Ningxia Universities (No. NYG-2022033), and the Key R&D Project of Autonomous Region (No. 2021BEG02037).
Author information
Authors and Affiliations
Contributions
JQH and LZB developed the search searches, extracted the data, assessed the study quality, performed the statistical analysis, and wrote the manuscript. YJB, YY and TZQ conceived and designed the study. WZQ and GXF cross-checked the collection and organization of data. All the authors revised and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Gregorio Milani
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lan, Z., Yan, J., Yang, Y. et al. Effectiveness of COVID-19 vaccines among children and adolescents against SARS-CoV-2 variants: a meta-analysis. Eur J Pediatr 182, 5235–5244 (2023). https://doi.org/10.1007/s00431-023-05216-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-05216-5