Abstract
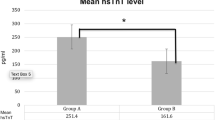
The purpose of the study is to test whether NT-proBNP serves as a screening tool for low-risk patent ductus arteriosus and safely avoids routine early echocardiography. This is a prospective observational study in preterm infants ≤32 weeks of gestational age. Infants with ≥5100 pg/ml (positive screening) at 48–72 hours of life received comprehensive echocardiography and were treated according to shunt severity. Infants with NT-proBNP below 5100 pg/ml (negative screening) were managed expectantly. The main outcome was need for ductus treatment within the first 7 days of life. One hundred twenty-five infants were included; 82 had a negative NT-proBNP screening and 43 had a positive NT-proBNP screening. No infant (0%) with a negative screening was treated for ductus while 26 (60.4%) with a positive screening were treated (p < 0.001). NT-proBNP avoided a 65.6% of routine echocardiograms. NT-proBNP had an excellent performance to predict PDA treatment (AUC = 0.967).
Conclusion: NT-proBNP at 48–72 hours of life has an excellent performance to detect low risk and avoids unnecessary echocardiograms. This may contribute to optimize PDA management in terms of resource utilization.
What is Known: • Patent ductus arteriosus is associated with significant morbidity in preterm infants. • Early echocardiography screening and individualized treatment of high-risk preterm infants may improve outcomes but is resource consuming. | |
What is New: • NT-proBNP can be used as a screening tool to detect low-risk patent ductus arteriosus. • Using NT-proBNP as a screening tool safely avoids a significant proportion of routine echocardiography and may improve resource utilization. |


Similar content being viewed by others
Availability of data and material
Full data are available upon reasonable request directly from the authors.
References
Mitra S, Scrivens A, von Kursell AM, Disher T (2020) Early treatment versus expectant management of hemodynamically significant patent ductus arteriosus for preterm infants. Cochrane Database Syst Rev 12:CD013278. https://doi.org/10.1002/14651858.CD013278.pub2
Mitra S, McNamara PJ (2020) Patent ductus arteriosus-time for a definitive trial. Clin Perinatol 47:617–639. https://doi.org/10.1016/j.clp.2020.05.007
Juszczak E, Gupta S (2018) Continued uncertainty regarding treatment of patent ductus arteriosus in premature infants and the role of clinical trials. Semin Fetal Neonatal Med 23:267–272. https://doi.org/10.1016/j.siny.2018.03.004
Hundscheid T, Jansen EJS, Onland W et al (2021) Conservative management of patent ductus arteriosus in preterm infants-a systematic review and meta-analyses assessing differences in outcome measures between randomized controlled trials and cohort studies. Front Pediatr 9:626261. https://doi.org/10.3389/fped.2021.626261
Sung SI, Chang YS, Ahn SY et al (2020) Conservative non-intervention approach for hemodynamically significant patent ductus arteriosus in extremely preterm infants. Front Pediatr 8:605134. https://doi.org/10.3389/fped.2020.605134
Weisz DE, McNamara PJ, El-Khuffash A (2017) Cardiac biomarkers and haemodynamically significant patent ductus arteriosus in preterm infants. Early Hum Dev 105:41–47. https://doi.org/10.1016/j.earlhumdev.2016.12.007
Schena F, Francescato G, Cappelleri A et al (2015) Association between hemodynamically significant patent ductus arteriosus and bronchopulmonary dysplasia. J Pediatr 166:1488–1492. https://doi.org/10.1016/j.jpeds.2015.03.012
Evans N, Gournay V, Cabanas F et al (2011) Point-of-care ultrasound in the neonatal intensive care unit: international perspectives. Semin Fetal Neonatal Med 16:61–68. https://doi.org/10.1016/j.siny.2010.06.005
Singh Y, Roehr CC, Tissot C et al (2018) Education, training, and accreditation of Neonatologist Performed Echocardiography in Europe-framework for practice. Pediatr Res 84:13–17. https://doi.org/10.1038/s41390-018-0078-9
Farombi-Oghuvbu I, Matthews T, Mayne PD et al (2008) N-terminal pro-B-type natriuretic peptide: a measure of significant patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed 93:F257-260. https://doi.org/10.1136/adc.2007.120691
El-Khuffash AF, Slevin M, McNamara PJ, Molloy EJ (2011) Troponin T, N-terminal pro natriuretic peptide and a patent ductus arteriosus scoring system predict death before discharge or neurodevelopmental outcome at 2 years in preterm infants. Arch Dis Child Fetal Neonatal Ed 96:F133-137. https://doi.org/10.1136/adc.2010.185967
Gokulakrishnan G, Kulkarni M, He S et al (2022) Brain natriuretic peptide and N-terminal brain natriuretic peptide for the diagnosis of haemodynamically significant patent ductus arteriosus in preterm neonates. Cochrane Database Syst Rev 12:CD013129. https://doi.org/10.1002/14651858.CD013129.pub2
Kulkarni M, Gokulakrishnan G, Price J et al (2015) Diagnosing significant PDA using natriuretic peptides in preterm neonates: a systematic review. Pediatrics 135:e510-525. https://doi.org/10.1542/peds.2014-1995
Evans N (2015) Preterm patent ductus arteriosus: a continuing conundrum for the neonatologist? Semin Fetal Neonatal Med 20:272–277. https://doi.org/10.1016/j.siny.2015.03.004
Sehgal A (2011) Haemodynamically unstable preterm infant: an unresolved management conundrum. Eur J Pediatr 170:1237–1245. https://doi.org/10.1007/s00431-011-1435-4
Rodriguez-Gonzalez M, Perez-Reviriego AA, Castellano-Martinez A (2020) Current role of cardiac biomarkers in extra-cardiac diseases in children. Biomark Med 14:1183–1187. https://doi.org/10.2217/bmm-2020-0232
Xiong T, Kulkarni M, Gokulakrishnan G et al (2020) Natriuretic peptides in bronchopulmonary dysplasia: a systematic review. J Perinatol 40:607–615. https://doi.org/10.1038/s41372-019-0588-2
Fritz AS, Keller T, Kribs A, Hünseler C (2021) Reference values for N-terminal pro-brain natriuretic peptide in premature infants during their first weeks of life. Eur J Pediatr 180:1193–1201. https://doi.org/10.1007/s00431-020-03853-8
Rodriguez-Blanco S, Oulego-Erroz I, Gautreaux-Minaya S et al (2018) Early NT-proBNP levels as a screening tool for the detection of hemodynamically significant patent ductus arteriosus during the first week of life in very low birth weight infants. J Perinatol 38:881–888. https://doi.org/10.1038/s41372-018-0123-x
García P, San Feliciano L, Benito F et al (2013) Outcome at two years corrected age of a cohort of very low birth weight infants from hospitals within the neonatal SEN1500 network. An Pediatr (Barc) 79:279–287. https://doi.org/10.1016/j.anpedi.2013.03.017
Barnes SC, Collinson PO, Galasko G et al (2004) Evaluation of N-terminal pro-B type natriuretic peptide analysis on the Elecsys 1010 and 2010 analysers. Ann Clin Biochem 41:459–463. https://doi.org/10.1258/0004563042466848
Oulego Erroz I, Alonso Quintela P, Jiménez Gonzalez A et al (2018) Impact of screening and treatment of low systemic blood flow in the prevention of severe intraventricular haemorrhage and/or death in pre-term infants. An Pediatr (Barc) 89:369–377. https://doi.org/10.1016/j.anpedi.2018.02.010
Mitra S, Florez ID, Tamayo ME et al (2018) Association of placebo, indomethacin, ibuprofen, and acetaminophen with closure of hemodynamically significant patent ductus arteriosus in preterm infants: a systematic review and meta-analysis. JAMA 319:1221–1238. https://doi.org/10.1001/jama.2018.1896
Hundscheid T, Onland W, Kooi EMW et al (2023) Expectant management or early ibuprofen for patent ductus arteriosus. N Engl J Med 388:980–990. https://doi.org/10.1056/NEJMoa2207418
Giesinger RE, Hobson AA, Bischoff AR et al (2023) Impact of early screening echocardiography and targeted PDA treatment on neonatal outcomes in “22–23” week and “24–26” infants. Semin Perinatol 47:151721. https://doi.org/10.1016/j.semperi.2023.151721
Rozé JC, Cambonie G, Marchand-Martin L et al (2015) Association between early screening for patent ductus arteriosus and in-hospital mortality among extremely preterm infants. JAMA 313:2441–2448. https://doi.org/10.1001/jama.2015.6734
King BC, Hagan J, Richardson T et al (2023) Hospital variation in neonatal echocardiography among very preterm infants at US children’s hospitals. J Perinatol 43:181–186. https://doi.org/10.1038/s41372-022-01522-2
Khan SS, Sithisarn T, Bada HS et al (2017) Urinary NT-proBNP levels and echocardiographic parameters for patent ductus arteriosus. J Perinatol 37:1319–1324. https://doi.org/10.1038/jp.2017.139
Tosse V, Pillekamp F, Verde P et al (2012) Urinary NT-proBNP, NGAL, and H-FABP may predict hemodynamic relevance of patent ductus arteriosus in very low birth weight infants. Neonatology 101:260–266. https://doi.org/10.1159/000334826
Author information
Authors and Affiliations
Contributions
GLB and IOE conceived and designed the study, analyzed data, drafter the manuscript and are fully accountable for all aspects of the manuscript. APV, CMG, PAQ, SRB, APM, MCP made substantial contributions to the design, acquired data, interpreted data, critically reviewed the manuscript and are fully accountable for all aspects of the present work. All authors gave their approval to the final version.
Corresponding author
Ethics declarations
Ethics approval
The present study has been approved by the local Institutional Review Board (Comité Ético de Investigation de las Áreas de León y El Bierzo).
Consent to participate
Parents or legal guardians gave their informed consent for participation. The study was performed in accordance with the last version of the Declaratión of Helsinki.
Competing interests
The authors declare no competing interests.
Disclaimer
The present work is original and has not been previously published nor it is being considered for publication elsewhere.
Additional information
Communicated by: Daniele De Luca
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
López-Blanco, G., Oulego-Erroz, I., Pou-Blázquez, Á. et al. NT-PROBNP as a screening tool for low-risk patent ductus arteriousus: a follow-up validation study. Eur J Pediatr 182, 5465–5471 (2023). https://doi.org/10.1007/s00431-023-05213-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-05213-8