Abstract
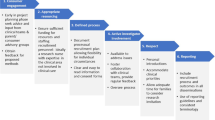
In pediatric oncology there are few examples of successful recruitment and retention strategies in psychosocial care research. This study aims to summarize experiences, challenges, and strategies for conducting randomized controlled trials (RCTs) of psychosocial intervention studies among children with cancer and their parent(s). We conducted a collective case study. To identify the cases, Pubmed and two trial registries were searched for ongoing and finished RCTs of psychosocial intervention studies for children with cancer and their parents. Online semi-structured expert interviews discussing recruitment and retention challenges and strategies were performed with principal investigators and research staff members of the identified cases. Nine studies were identified. Investigators and staff from seven studies participated, highlighting challenges and strategies within three major themes: eligibility, enrollment and retention. Regarding eligibility, collaborating constructively with healthcare professionals and involving them before the start of the study were essential. Being flexible, training the research staff, enabling alignment with the participants’ situation, and providing consistency in contact between the research staff member and the families were important strategies for optimizing enrollment and retention. All studies followed a stepped process in recruitment.
Conclusion: Although recruitment and retention in some selected studies were successful, there is a paucity of evidence on experienced recruitment and retention challenges in pediatric psychosocial research and best practices on optimizing them. The strategies outlined in this study can help researchers optimize their protocol and trial-implementation, and contribute to better psychosocial care for children with cancer and their parents.
Trial Registration: This study is not a clinical trial.
What is Known: • Performing RCTs is challenging, particularly in pediatric psychosocial research when both the child and parent are targeted. Recruitment and retention are common concerns. In pediatric oncology, there are few examples of successful recruitment and retention strategies in psychosocial care research. | |
What is New: • Key strategies to collaborate constructively with healthcare professionals were outlined. Being flexible, training the research staff, alignment with the participant’s situations and providing consistency in contact between the research staff member and the families were considered as essential strategies. |

Similar content being viewed by others
Data availability
Data are available from the corresponding author on reasonable request.
Abbreviations
- pACP:
-
Pediatric Advance Care Planning
- RCT:
-
Randomized controlled trial
- SWAT:
-
Study Within A Trial
References
Houghton C, Dowling M, Meskell P, Hunter A, Gardner H, Conway A, Treweek S, Sutcliffe K, Noyes J, Devane D et al (2020) Factors that impact on recruitment to randomised trials in health care : a qualitative evidence synthesis. Cochrane Database Syst Rev 10:1–20
Kazak AE, Simms S, Alderfer MA, Rourke MT, Crump T, McClure K, Jones P, Rodriquez A, Boeving A, Hwang W et al (2005) Feasibility and preliminary outcomes from a pilot study of a brief psychological intervention for families of children newly diagnosed with cancer. J Pediatr Psychol 30(8):644–655
Saarijärvi M, Wallin L, Moons P, Gyllensten H, Bratt EL (2020) Factors affecting adolescents’ participation in randomized controlled trials evaluating the effectiveness of healthcare interventions: The case of the STEPSTONES project. BMC Med Res Methodol 20(1):1–11
Kling J, Nordgreen T, Kvalem IL, Williamson H, Feragen KB (2021) Recruiting hard-to-engage groups to online psychosocial interventions: Experiences from an RCT study targeting adolescents with a visible difference. Contemp Clin Trials Commun 24:100869
Karlson CW, Rapoff MA (2009) Attrition in randomized controlled trials for pediatric chronic conditions. J Pediatr Psychol 34(7):782–793
Bradford N, Cashion C, Condon P, Rumble S, Bowers A (2021) Recruitment principles and strategies for supportive care research in pediatric oncology. BMC Med Res Methodol 21(1):1–9
Stehl ML, Kazak AE, Alderfer MA, Rodriguez A, Hwang WT, Pai ALH, Boeving A, Reilly A (2009) Conducting a randomized clinical trial of an psychological intervention for parents/caregivers of children with cancer shortly after diagnosis. J Pediatr Psychol 34(8):803–816
Wakefield CE, Fardell JE, Doolan EL, Aaronson NK, Jacobsen PB, Cohn RJ, King M (2017) Participation in psychosocial oncology and quality-of-life research: a systematic review. Lancet Oncology 18:e153–e165
Johnston D, Gerbing R, Alonzo T, Aplenc R, Nagarajan R, Schulte F, Cullen P, Sung L (2015) Patient-reported outcome coordinator did not improve quality of life assessment response rates: A report from the children’s oncology group. PLoS One 10(4)
Hou SHJ, Stokoe M, Zwicker H, Young-Speirs M, Pelletier W, Guilcher GMT, Khu M, Schulte F (2023) Pediatric Hematopoietic Cell Transplantation: A Longitudinal Assessment of Health-Related Quality of Life of Pediatric Donors. J Clin Psychol Med Settings 1–10
Hudson BF, Oostendorp LJM, Candy B, Vickerstaff V, Jones L, Lakhanpaul M, Bluebond-Langner M, Stone P (2017) The under reporting of recruitment strategies in research with children with life-threatening illnesses: A systematic review. Palliat Med 31(5):419–436
Treweek S, Lockhart P, Pitkethly M, Cook JA, Kjeldstrøm M, Johansen M, Taskila TK, Sullivan FM, Wilson S, Jackson C et al (2013) Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open 3(2)
Crowe S, Cresswell K, Robertson A, Huby G, Avery A, Sheikh A (2011) The case study approach. BMC Med Res Methodol 11(1):100
Forsman AK, Nordmyr J, Wahlbeck K (2011) Psychosocial interventions for the promotion of mental health and the prevention of depression among older adults. Health Promot Int 26(1):85–107
Braun V, Clarke V (2006) Using thematic analysis in psychology. Qual Res Psychol 3(2):77–101
Cheng KKF, Tan LML (2021) A pilot study of the effect of a home-based multimodal symptom-management program in children and adolescents undergoing chemotherapy. Cancer Rep 4(3):1–9
Zhang P, Mo L, Torres J, Huang X (2019) Effects of cognitive behavioral therapy on psychological adjustment in Chinese pediatric cancer patients receiving chemotherapy: A randomized trial. Med 98(27)
Kotecha RS, Kees UR, Cole CH, Gottardo NG (2015) Rare childhood cancers-an increasing entity requiring the need for global consensus and collaboration. Cancer Med 4(6):819–824
Northouse L, Rosset T, Phillips L, Mood D, Schafenacker A, Kershaw T (2006) Research with families facing cancer: the challenges of accrual and retention. Res Nurs Health 29:199–211
Hanson LC, Bull J, Wessell K, Massie L, Bennett RE, Kutner JS, Aziz NM, Abernethy A (2014) Strategies to support recruitment of patients with life-limiting illness for research: The palliative care research cooperative group. J Pain Symptom Manage 48(6):1021–1030
Lohani M, Hendershot K, Pelletier W, Stegenga K, Dixon M, Hinds P, Alderfer M, Pentz R (2018) Potential Benefits to Families, Children, and Adolescents, Enrolled in Longitudinal Qualitative Research. IRB: Ethics & Human Res 40(4)
Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, Boyd KA, Craig N, French DP, McIntosh E et al (2021) A new framework for developing and evaluating complex interventions: Update of Medical Research Council guidance. BMJ 374(2018):1–11
Beets MW, Weaver RG, Ioannidis JPA, Geraci M, Brazendale K, Decker L, Okely AD, Lubans D, van Sluijs E, Jago R (2020) Identification and evaluation of risk of generalizability biases in pilot versus efficacy/effectiveness trials: A systematic review and meta-analysis. Int J Behav Nutr Phys Act 17(1):1–20
Moore GF, Evans RE, Hawkins J, Littlecott H, Melendez-Torres GJ, Bonell C (2019) From complex social interventions to interventions in complex social systems: Future directions and unresolved questions for intervention development and evaluation. Evaluation 25(1):23–45
Burchett HED, Kneale D, Blanchard L, Thomas J (2020) When assessing generalisability, focusing on differences in population or setting alone is insufficient. Trials 21(1):1–4
Czwikla J, Herzberg A, Kapp S, Kloep S, Rothgang H, Nitschke I, Haffner C, Hoffmann F (2022) Generalizability and reach of a randomized controlled trial to improve oral health among home care recipients: comparing participants and nonparticipants at baseline and during follow-up. Trials 23(1):1–13
Canter KS, Vega G, Ramirez AP, Deatrick JE, Kazak AE (2020) Strategies for Successful Recruitment and Retention of Parents in Pediatric Psychosocial eHealth Interventions: A Qualitative Study in Pediatric Oncology. J Pediatr Psychol 45(5):530–539
Treweek S, Bevan S, Bower P, Campbell M, Christie J, Clarke M, Collett C, Cotton S, Devane D, El Feky A et al (2018) Trial Forge Guidance 1: What is a Study Within A Trial (SWAT)? Trials 19(1):1–5
Martin-Kerry J, Parker A, Bower P, Watt I, Treweek S, Torgerson D et al (2019) SWATted away: The challenging experience of setting up a programme of SWATs in paediatric trials. Trials 20(1):1–6
Rosenberg AR, Bradford MC, Barton KS, Etsekson N, McCauley E, Curtis JR, Wolfe J, Baker KS, Yi-Frazier Y (2019) Hope and benefit finding: results from the PRISM randomized controlled trial. Pediatr Blood Cancer 66(1):e27485
Barrera M, Desjardins L, Prasad S, Shama W, Alexander S, Szatmari P, Hancock K (2021) Pilot randomized psychosocial trial of a screening intervention in pediatric oncology. Psychooncology 31(5):735–744
Wolfe J, Orellana L, Cook EF, Ullrich C, Kang T, Geyer JR, Feudtner C, Weeks JC, Dussel V (2014) Improving the care of children with advanced cancer by using an electronic patient-reported feedback intervention: Results from the PediQUEST randomized controlled trial. J Clin Oncol 32(11):1119–1126
Wolfe J, Dussel V (2018) The PediQUEST Response Intervention Study. Clinical Trials Gov Available from: https://clinicaltrials.gov/ct2/show/NCT03408314
Requena ML, Avery M, Feraco AM, Uzal LG, Wolfe J, Dussel V (2022) Normalization of Symptoms in Advanced Child Cancer: The PediQUEST-Response Case Study. J Pain Symptom Manage 63(4):548–562
Thompkins JD, Needle J, Baker JN, Briggs L, Cheng YI, Wang J, Friebert S, Lyon ME (2021) Pediatric advance care planning and families’ positive caregiving appraisals: An RCT. Pediatrics 147(6)
Akard TF, Dietrich MS, Friedman DL, Wray S, Gerhardt CA, Hendricks-Ferguson V, Hinds PS, Rhoten B, Gilmer MJ (2021) Randomized Clinical Trial of a Legacy Intervention for Quality of Life in Children with Advanced Cancer. J Palliat Med 24(5):680–688
Salem H, Kazak AE, Andersen EW, Belmonte F, Johansen C, Schmiegelow K, Winther JF, Whener PK, Hasle H, Rosthøj S et al (2021) Home-based cognitive behavioural therapy for families of young children with cancer (FAMOS): A nationwide randomised controlled trial. Pediatr Blood Cancer 68:e28853
van Driessche A, Gilissen J, De Vleminck A, Kars M, Fahner J, van der Werff ten Bosch J, Deliens L, Cohen J, Beernaert K (2022) The BOOST paediatric advance care planning intervention for adolescents with cancer and their parents: development, acceptability and feasibility. BMC Pediatr 22(1)
van Driessche A, De Vleminck A, Gilissen J, Kars MC, Van Der Werff ten Bosch J, Deliens L, Cohen J, Beernaert K (2021) Advance care planning for adolescents with cancer and their parents : study protocol of the BOOST pACP multi-centre randomised controlled trial and process evaluation 21(376):1–16
Acknowledgments
Thank you to research staff members Alison O’Daffer, Victoria Klein, Kelly Hancock, Hanin Salem, Jessica Thompkins, Sarah Friebert, Madeline Avery, Aurelie Joos and Kim Eecloo for their invaluable input on experienced recruitment challenges and strategies to overcome them. We would like to thank William Wright for language editing.
Funding
This study was supported by The Research Foundation – Flanders (FWO) [under grant Agreement G.0194.18 N] and Kinderkankerfonds [no grant number].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Anne van Driessche performed the interviews and analysis, drafted the initial manuscript, and adapted the manuscript. Prof Kim Beernaert, Prof Joachim Cohen, Prof Luc Deliens, Dr Marijke C. Kars and Prof Aline De Vleminck critically reviewed and revised the manuscript. Dr Maureen E. Lyon, Dr Maru Barrera, Dr Veronica Dussel, Dr Pernille Bidstrup, Dr Abby R. Rosenberg and Dr Terrah F. Akard participated in the study as a respondent sharing expertise on the topic of interest, and critically reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The project this study is part of was approved by the Medical Ethics Committee of the University Hospital of Brussels in Flanders, Belgium (B1432020000177). The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments and in compliance with our institutional guidelines.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
All respondents provided informed consent for publication of the study characteristics and use of quotes.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Peter de Winter
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Joachim Cohen and Aline De Vleminck shared last authorship.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
van Driessche, A., Beernaert, K., Deliens, L. et al. Recruitment and retention challenges and strategies in randomized controlled trials of psychosocial interventions for children with cancer and their parents: a collective case study. Eur J Pediatr 182, 4683–4706 (2023). https://doi.org/10.1007/s00431-023-05139-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-05139-1