Abstract
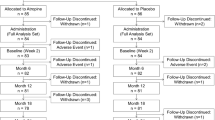
This study aims to evaluate the efficacy of 0.01% atropine eye drops in preventing myopia shift and myopia onset in premyopic children. A prospective, randomized, double-masked, placebo-controlled, and crossover trial was conducted over 13 months. Sixty premyopic children aged 6–12 years with cycloplegic spherical equivalent refraction (SER) > − 0.75 D and ≤ + 0.50 D in both eyes were assigned in a 1:1 ratio to receive one drop of 0.01% atropine or placebo once nightly for 6 months (period 1), followed by a 1-month recovery period. Then, the 0.01% atropine group was crossed over to the placebo group, and the latter was crossed over to the 0.01% atropine group for another 6 months (period 2). The primary outcomes were changes in SER and axial length (AL), and the secondary outcomes were the proportion of myopia onset (SER ≤ − 0.75D) and fast myopic shift (change in SER ≤ − 0.25D) in the two periods. Generalized estimating equation (GEE) model performed a statistically significant treatment effect of 0.01% atropine compared with placebo (pSER = 0.02, pAL < 0.001), with a mean SER and AL difference of 0.20D (− 0.15 ± 0.26D vs. − 0.34 ± 0.34D) and 0.11 mm (0.17 ± 0.11 mm vs. 0.28 ± 0.14 mm) in period 1, and 0.17D (− 0.18 ± 0.24D vs. − 0.34 ± 0.31D) and 0.10 mm (0.15 ± 0.15 mm vs. 0.24 ± 0.11 mm) in period 2. The GEE model showed that the proportion of myopia onset (p = 0.004) and fast myopic shift (p = 0.009) was significantly lower in the 0.01% atropine group than that in the placebo group. The period effect was not statistically significant (all p > 0.05). A total of 0.01% atropine significantly prevented myopic shift, axial elongation, and myopia onset in premyopic schoolchildren in central Mainland China.
Conclusion: Within the limits of only two consecutive 6-month observation period, 0.01% atropine eye drops effectively prevented myopic shift, axial elongation, and myopia onset in premyopic children.
Trial registration: This trial was registered in the Chinese Clinical Trial Registry (Registration number: ChiCTR2000034760). Registered 18 July 2020.
What is Known: • Minimal studies on interventions for pre-myopia, despite the International Myopia Institute stating that preventing myopia is an “even more valuable target” for science and practice than reducing progression after onset. | |
What is New: • A total of 0.01% atropine eye drops may safely and effectively reduce the proportion of myopia onset and fast myopic shift in premyopic schoolchildren. |





Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are not publicly available due to [REASON(S) WHY DATA ARE NOT PUBLIC].
Abbreviations
- AMP:
-
Accommodation amplitude
- AL:
-
Axial length
- GEE:
-
Generalized estimating equation
- PD:
-
Pupil diameter
- Reg:
-
Regression
- SER:
-
Spherical equivalent refraction
References
Fredrick DR (2002) Myopia. Br Med J 324:1195–1199. https://doi.org/10.1136/bmj.324.7347.1195
Parssinen O, Kauppinen M, Viljanen A (2014) The progression of myopia from its onset at age 8–12 to adulthood and the influence of heredity and external factors on myopic progression. A 23-year follow-up study. Acta Ophthalmol 92:730–739. https://doi.org/10.1111/aos.12387
Hu Y, Ding XH, Guo XX, Chen YX, Zhang J, He MG (2020) Association of age at myopia onset with risk of high myopia in adulthood in a 12-year follow-up of a Chinese Cohort. JAMA Ophthalmol 138:1129–1134. https://doi.org/10.1001/jamaophthalmol.2020.3451
Parssinen O, Kauppinen M (2019) Risk factors for high myopia: a 22-year follow-up study from childhood to adulthood. Acta Ophthalmol 97:510–518. https://doi.org/10.1111/aos.13964
Flitcroft DI, He MG, Jonas JB, Jong M, Naidoo K, Ohno-Matsui K, Rahi J, Resnikoff S, Vitale S, Yannuzzi L (2019) IMI - Defining and classifying myopia: a proposed set of standards for clinical and epidemiologic studies. Invest Ophthalmol Vis Sci 60:M20–M30. https://doi.org/10.1167/iovs.18-25957
Mutti DO, Hayes JR, Mitchell GL et al (2007) Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Invest Ophthalmol Vis Sci 48:2510–2519. https://doi.org/10.1167/iovs.06-0562
Xiang F, He MG, Morgan IG (2012) Annual changes in refractive errors and ocular components before and after the onset of myopia in Chinese children. Ophthalmology 119:1478–1484. https://doi.org/10.1016/j.ophtha.2012.01.017
Wu PC, Chuang MN, Choi J, Chen H, Wu G, Ohno-Matsui K, Jonas JB, Cheung CMG (2019) Update in myopia and treatment strategy of atropine use in myopia control. Eye 33:3–13. https://doi.org/10.1038/s41433-018-0139-7
Modjtahedi BS, Abbott RL, Fong DS, Lum F, Tan D, Task Force M (2021) Reducing the global burden of myopia by delaying the onset of myopia and reducing myopic progression in children the academy’s task force on myopia. Ophthalmology 128:816–826. https://doi.org/10.1016/j.ophtha.2020.10.040
Li FF, Yam JC (2019) Low-concentration atropine eye drops for myopia progression. Asia-Pac J Ophthalmol 8:360–365. https://doi.org/10.1097/apo.0000000000000256
Yam JC, Jiang YN, Tang SM et al (2019) Low-concentration atropine for myopia progression (LAMP) study a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology 126:113–124. https://doi.org/10.1016/j.ophtha.2018.05.029
Yam JC, Li FF, Zhang XJ et al (2020) Two-year clinical trial of the low-concentration atropine for myopia progression (LAMP) study phase 2 report. Ophthalmology 127:910–919. https://doi.org/10.1016/j.ophtha.2019.12.011
Yam JC, Zhang XJ, Zhang YZ et al (2022) Three-year clinical trial of low-concentration atropine for myopia progression (LAMP) study: continued versus washout phase 3 report. Ophthalmology 129:308–321. https://doi.org/10.1016/j.ophtha.2021.10.002
Fu AC, Stapleton F, Wei L, Wang WQ, Zhao BX, Watt K, Ji N, Lyu Y (2020) Effect of low-dose atropine on myopia progression, pupil diameter and accommodative amplitude: low-dose atropine and myopia progression. Br J Ophthalmol 104:1535–1541. https://doi.org/10.1136/bjophthalmol-2019-315440
Del Valle IG, Alvarez-Lorenzo C (2021) Atropine in topical formulations for the management of anterior and posterior segment ocular diseases. Expert Opin Drug Deliv 18:1245–1259. https://doi.org/10.1080/17425247.2021.1909568
He MG, Xiang F, Zeng YF, Mai JC, Chen QY, Zhang J, Smith WN, Rose K, Morgan IG (2015) Effect of time spent outdoors at school on the development of myopia among children in China a randomized clinical trial. JAMA-J Am Med Assoc 314:1142–1148. https://doi.org/10.1001/jama.2015.10803
Huang HM, Chang DST, Wu PC (2015) The association between near work activities and myopia in children-a systematic review and meta-analysis. PLoS ONE 10:15. https://doi.org/10.1371/journal.pone.0140419
Jethani J (2022) Efficacy of low-concentration atropine (0.01%) eye drops for prevention of axial myopic progression in premyopes. Indian J Ophthalmol 70:238–240. https://doi.org/10.4103/ijo.IJO_1462_21
Fang PC, Chung MY, Yu HJ, Wu PC (2010) Prevention of myopia onset with 0.025% atropine in premyopic children. J Ocular Pharmacol Ther 26:341–345. https://doi.org/10.1089/jop.2009.0135
Hasebe S, Ohtsuki H, Nonaka T, Nakatsuka C, Miyata M, Hamasaki I, Kimura S (2008) Effect of progressive addition lenses on myopia progression in Japanese children: a prospective, randomized, double-masked, crossover trial. Invest Ophthalmol Vis Sci 49:2781–2789. https://doi.org/10.1167/iovs.07-0385
Swarbrick HA, Alharbi A, Watt K, Lum E, Kang P (2015) Myopia control during orthokeratology lens wear in children using a novel study design. Ophthalmology 122:620–630. https://doi.org/10.1016/j.ophtha.2014.09.028
Anstice NS, Phillips JR (2011) Effect of dual-focus soft contact lens wear on axial myopia progression in children. Ophthalmology 118:1152–1161. https://doi.org/10.1016/j.ophtha.2010.10.035
Wellek S, Blettner M (2012) On the proper use of the crossover design in clinical trials part 18 of a series on evaluation of scientific publications. Dtsch Arztebl Int 109:276–281. https://doi.org/10.3238/arztebl.2012.0276
Yu SA, Lv Y, Wang WQ, Cui C, Wei L, Huang CC, Ma NN, Zhao BX, Zhang JJ, Fu AC (2022) Effects of 0.01% atropine eye drops on the prevention of myopia onset among schoolchildren: a randomized, double-blind, controlled trial. Chinese J Exp Ophthalmol 40:533–540. https://doi.org/10.3760/cma.j.cn115989-20210821-00470
Rahi JS, Cumberland PM, Peckham CS (2011) Myopia over the lifecourse: prevalence and early life influences in the 1958 British Birth Cohort. Ophthalmology 118:797–804. https://doi.org/10.1016/j.ophtha.2010.09.025
Williams KM, Bertelsen G, Cumberland P et al (2015) Increasing prevalence of myopia in Europe and the impact of education. Ophthalmology 122:1489–1497. https://doi.org/10.1016/j.ophtha.2015.03.018
Wang M, Cui C, Sui Y, Yu SA, Ma JX, Fu AC (2022) Effect of 0.02% and 0.01% atropine on astigmatism: a two-year clinical trial. BMC Ophthalmol 22:9. https://doi.org/10.1186/s12886-022-02385-z
Cui C, Li XJ, Lyu Y, Wei L, Zhao BX, Yu SA, Rong JB, Bai YH, Fu AC (2021) Safety and efficacy of 0.02% and 0.01% atropine on controlling myopia progression: a 2-year clinical trial. Sci Rep 11:8. https://doi.org/10.1038/s41598-021-01708-2
Fu AC, Stapleton F, Li W, Wang WQ, Zhao BX, Watt K, Yu SA, Cui C, Lyu Y (2021) Risk factors for rapid axial length elongation with low concentration atropine for myopia control. Sci Rep 11:7. https://doi.org/10.1038/s41598-021-88719-1
Goyal R, North RV, Morgan JE (2003) Comparison of laser interferometry and ultrasound A-scan in the measurement of axial length. Acta Ophthalmol Scand 81:331–335. https://doi.org/10.1034/j.1600-0420.2003.00092.x
Rose LT, Moshegov CN (2003) Comparison of the Zeiss IOLMaster and applanation A-scan ultrasound: biometry for intraocular lens calculation. Clin Exp Ophthalmol 31:121–124. https://doi.org/10.1046/j.1442-9071.2003.00617.x
Wei SF, Li SM, An WZ, Du JL, Liang XT, Sun YY, Zhang DX, Tian JX, Wang NL (2020) Safety and efficacy of low-dose atropine eyedrops for the treatment of myopia progression in Chinese children a randomized clinical trial. JAMA Ophthalmol 138:1178–1184. https://doi.org/10.1001/jamaophthalmol.2020.3820
Chia A, Chua WH, Cheung YB, Wong WL, Lingham A, Fong A, Tan D (2012) Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (atropine for the treatment of myopia 2). Ophthalmology 119:347–354. https://doi.org/10.1016/j.ophtha.2011.07.031
Loughman J, Flitcroft DI (2016) The acceptability and visual impact of 0.01% atropine in a Caucasian population. Br J Ophthalmol 100:1525–1529. https://doi.org/10.1136/bjophthalmol-2015-307861
Kinoshita N, Konno Y, Hamada N, Kanda Y, Shimmura-Tomita M, Kaburaki T, Kakehashi A (2020) Efficacy of combined orthokeratology and 0.01% atropine solution for slowing axial elongation in children with myopia: a 2-year randomised trial. Sci Rep 10:11. https://doi.org/10.1038/s41598-020-69710-8
Tan Q, Ng ALK, Choy BNK, Cheng GPM, Woo VCP, Cho P (2020) One-year results of 0.01% atropine with orthokeratology (AOK) study: a randomised clinical trial. Ophthalmic Physiol Opt 40:557–566. https://doi.org/10.1111/opo.12722
Acknowledgements
We thank all the teenagers and their parents who participated in this study.
Funding
This work was supported by the Key Scientific Research Project of Universities of Henan Education Department (Grant number 22A320024).
Author information
Authors and Affiliations
Contributions
Study concept and design: Aicun Fu, Weiqun Wang, Fengyan Zhang, Junjie Zhang. Acquisition, analysis or interpretation of data: Aicun Fu, Shiao Yu, Nana Ma, Congcong Huang, Ming Wang, Li Wei. Revised paper for important intellectual content and final approval of the version submitted for publication: Aicun Fu, Shiao Yu, Weiqun Wang, Fengyan Zhang. Study supervision: Aicun Fu. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Human Ethics Committee of the First Affiliated Hospital of Zhengzhou University (Number: 2020-KY-286).
Consent to participate
Written informed consent was obtained from all individual participants and their parents.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Gregorio Milani.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, W., Zhang, F., Yu, S. et al. Prevention of myopia shift and myopia onset using 0.01% atropine in premyopic children — a prospective, randomized, double-masked, and crossover trial. Eur J Pediatr 182, 2597–2606 (2023). https://doi.org/10.1007/s00431-023-04921-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-04921-5