Abstract
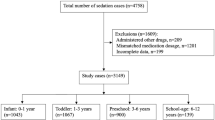
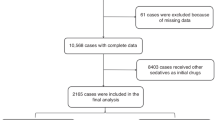
The purpose of this study is to audit the efficacy and safety of intranasal dexmedetomidine sedation for non-invasive procedural sedation in children provided by nurses of the procedural sedation (PROSA) team in the University Hospitals Leuven. Efficacy (successful sedation as sole sedative) and safety (cardiorespiratory monitoring, saturation) were assessed. In this audit, prospectively recorded data were extracted from the medical files in 772 patients between 4 weeks to 18 years old, who underwent sedation with intranasal dexmedetomidine (2–4 µg/kg) by the nurse-driven PROSA team, following pre-screening on risk factors. Ninety-one percent of the patients were successfully sedated (single dose, monotherapy), 60 patients (7.8%) needed an additional intervention during sedation, 37 (4.8%) needed an extra dose of intranasal dexmedetomidine, and 14 (1.8%) received an additional other sedative. Successful sedation rates were higher in younger children, and medical imaging was the most common indication. Sedation failed in 12 (1.6%) patients, with 10 of them failed to fall asleep. Adverse events were limited in number (n = 13, 1.7%) and severity: 4 patients had a low heart rate (one received atropine), one had an irregular heart rate, and 7 desaturation events were described. Hypotension was treated with normal saline in one case.
Conclusions: In this nurse-driven PROSA setting, intranasal dexmedetomidine is effective and safe for non-invasive procedural sedation in an a priori low risk group of paediatric patients.
What is Known: • Procedural sedation outside the operating theatre or intensive care units is increasingly used, including sedation performed by non-anaesthesiologists or nurses. This resulted in the development of procedural sedation and analgesia (PROSA) teams. • Off-label use of intranasal dexmedetomidine in children is increasing, with a limited number of audits on this practice, its safety and efficacy. | |
What is New: • In an audit on 772 procedures, nurse-driven intranasal dexmedetomidine administration as sedation for non-invasive procedures in children within a structured framework was safe and effective. • Imaging (CT, MRI) was the most common procedural indication in our study, but also nuclear imaging techniques were included. |
Similar content being viewed by others
Availability of data and materials
The data and materials are available and can be provided by the corresponding author, if based upon reasonable request and based on a study protocol.
Abbreviations
- ASA classification:
-
American Society of Anesthesiologists’ classification of Physical Health
- CT scan:
-
Computerised tomography scan
- DMSA scan:
-
Dimercaptosuccinic acid scan
- ECG:
-
Electrocardiogram
- EC scan:
-
Ethylene dicysteine scan
- IV:
-
Intravenous
- MIBG scan:
-
Meta-iodobenzylguanidine scan
- MRI:
-
Magnetic resonance imaging
- PAC puncture:
-
Port-A-Cath puncture
- PET scan:
-
Positron emission tomography scan
- PO:
-
Per Os
- PROSA:
-
Procedural sedation and analgesia
- SD:
-
Standard deviation
References
Kerkhofs L, Allegaert K, Toelen J, Vanhonsebrouck K (2022) Pediatric procedural sedation and analgesia (PROSA) in the Leuven University Hospitals: an audit on efficacy and safety. Children 9:776
Ambi U, Joshi C, Ganeshnavar A, Adrash E (2012) Intranasal dexmedetomidine for paediatric sedation for diagnostic magnetic resonance imaging studies. Indian J Anaesth 56:587
Li L, Zhou J, Yu D, Hao X, Xie Y, Zhu T (2020) Intranasal dexmedetomidine versus oral chloral hydrate for diagnostic procedures sedation in infants and toddlers: a systematic review and meta-analysis. Medicine (Baltimore) 99:e19001
Cozzi G, Norbedo S, Barbi E (2017) Intranasal dexmedetomidine for procedural sedation in children, a suitable alternative to chloral hydrate. Paediatr Drugs 19:107–111
Pershad J, Palmisano P, Nichols M (1999) Chloral hydrate: the good and the bad. Pediatr Emerg Care 15:432–435
Haselkorn T, Whittemore AS, Udaltsova N, Friedman GD (2006) Short-term chloral hydrate administration and cancer in humans. Drug Saf 29:67–77
Fan L, Lim Y, Wong GS, Taylor R (2021) Factors affecting successful use of intranasal dexmedetomidine: a cohort study from a national paediatrics tertiary centre. Transl Pediatr 10:765–772
Behrle N, Birisci E, Anderson J, Schroeder S, Dalabih A (2017) Intranasal dexmedetomidine as a sedative for pediatric procedural sedation. J Pediatr Pharmacol Ther 22:4–8
Miller JW, Divanovic AA, Hossain MM, Mahmoud MA, Loepke AW (2016) Dosing and efficacy of intranasal dexmedetomidine sedation for pediatric transthoracic echocardiography: a retrospective study. Can J Anaesth 63:834–841
Mondardini MC, Amigoni A, Cortellazzi P, Di Palma A, Navarra C, Picardo SG, Puzzutiello R, Rinaldi L, Vitale F, Zito Marinosci G et al (2019) Intranasal dexmedetomidine in pediatrics: update of current knowledge. Minerva Anestesiol 85:1334–1345
Lin Y, Zhang R, Shen W, Chen Q, Zhu Y, Li J, Chi W, Gan X (2020) Dexmedetomidine versus other sedatives for non-painful pediatric examinations: a systematic review and meta-analysis of randomized controlled trials. J Clin Anesth 62:109736
Tervonen M, Pokka T, Kallio M, Peltoniemi O (2020) Systematic review and meta-analysis found that intranasal dexmedetomidine was a safe and effective sedative drug during paediatric procedural sedation. Acta Paediatr 109:2008–2016
European Medicines Agency (2016) Annex 1, Summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/dexdor-epar-product-information_en.pdf. Accessed 18 Aug 2022
Mason KP, Lerman J (2011) Dexmedetomidine in children. Anesth Analg 113:1129–1142
Li A, Yuen VM, Goulay-Dufaÿ S, Sheng Y, Standing JF, Kwok PCL, Leung MKM, Leung AS, Wong ICK, Irwin MG (2018) Pharmacokinetic and pharmacodynamic study of intranasal and intravenous dexmedetomidine. Br J Anaesth 120:960–968
Mason KP, Zgleszewski SE, Dearden JL, Dumont RS, Pirich MA, Stark CD, D’Angelo P, Macpherson S, Fontaine PJ, Connor L et al (2006) Dexmedetomidine for pediatric sedation for computed tomography imaging studies. Anesth Analg 103:57–62
Yuen VM, Li BL, Cheuk DK, Leung MKM, Hui TWC, Wong IC, Lam WW, Choi SW, Irwin MG (2017) A randomised controlled trial of oral chloral hydrate vs. intranasal dexmedetomidine before computerised tomography in children. Anaesthesia 72:1191–1195
Miller J, Xue B, Hossain M, Zhang MZ, Loepke A, Kurth D (2016) Comparison of dexmedetomidine and chloral hydrate sedation for transthoracic echocardiography in infants and toddlers: a randomized clinical trial. Paediatr Anaesth 26:266–272
Sury MR, Hatch DJ, Deeley T, Dicks-Mireaux CWK (1999) Development of a nurse-led service for paediatric magnetic resonance imaging. Lancet 353:1667–1671
Schrier L, Hadjipanayis A, Stiris T, Ross-Russell RI, Valiulis A, Turner MA, Zhao W, De Cock P, de Wildt SN, Allegaert K et al (2020) Off-label use of medicines in neonates, infants, children, and adolescents: a joint policy statement by the European Academy of Paediatrics and the European Society for Developmental Perinatal and Pediatric Pharmacology. Eur J Pediatr 179:839–847
Filho EM, Robinson F, De Carvalho WB, Gilio AE, Mason KP (2015) Intranasal dexmedetomidine for sedation for pediatric computed tomography imaging. J Pediatr 166:1313–1315
Jackson TJ, Dawes D, Ahmad S, Martin D, Gyamtso C (2022) Dexmedetomidine improves success of paediatric MRI sedation. Arch Dis Child 107:692–694
Lewis J, Bailey CR (2020) Intranasal dexmedetomidine for sedation in children; a review. J Perioper Pract 30:170–175
Sulton C, Kamat P, Mallory M, Reynolds J (2020) The use of intranasal dexmedetomidine and midazolam for sedated magnetic resonance imaging in children. Pediatr Emerg Care 36:138–142
Sulton C, McCracken C, Simon HK, Hebbar K, Reynolds J, Cravero J, Mallory M, Kamat P (2016) pediatric procedural sedation using dexmedetomidine: a report from the pediatric sedation research consortium. Hosp Pediatr 6:536–544
Chiaretti A, Barone G, Rigante D, Ruggiero A, Pierri F, Barbi E, Barone G, Riccardi R (2011) Intranasal lidocaine and midazolam for procedural sedation in children. Arch Dis Child 96:160–163
Del Pizzo J, Callahan JM (2014) Intranasal medications in pediatric emergency medicine. Pediatr Emerg Care 30:496–501
Van den Anker J, Reed MD, Allegaert K, Kearns GL (2018) Developmental changes in pharmacokinetics and pharmacodynamics. J Clin Pharmacol 58(Suppl 10):S10–S25
Mason K, Green M, Piacevoli Q, International Sedation Task Force (2012) Adverse event reporting tool to standardize the reporting and tracking of adverse events during procedural sedation: a consensus document from the World SIVA International Sedation Task Force. Br J Anaesth 108:13–20
Author information
Authors and Affiliations
Contributions
Emma Goyens (EG), Karel Allegaert (KA), Jaan Toelen (JT) and Koen Vanhonsebrouk (KV) planned and designed the study and ensured ethics approval. KV extracted the data and created the anonymized, blinded database. EG and KA wrote the first draft. JT, KV, Frederik Debuck (FD) and Julie Lauweryns (JL) critically revised the paper. All authors (EG, KA, FD, JL, JT, KV) participated in the discussion of the results and agreed on the final version of the paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Research Ethics Committee UZ/KU Leuven (MP016814). As audit based on a fully anonymized, blinded retrospective database analysis, consent to participate was not applicable, as supported by the Research Ethics Committee involved.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Gregorio Milani
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goyens, E., Allegaert, K., De Buck, F. et al. Nurse-driven intranasal dexmedetomidine administration as sedation for non-invasive procedures in children: a single centre audit. Eur J Pediatr 182, 899–905 (2023). https://doi.org/10.1007/s00431-022-04722-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-022-04722-2