Abstract
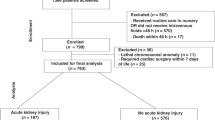
To investigate the risk factors for death in critically ill neonates receiving continuous renal replacement therapy (CRRT). This retrospective study analyzed the clinical data of critically ill neonates receiving CRRT at two tertiary hospitals from January 2015 to December 2021. A multi-factor logistic regression analysis was performed, and the predictive value of relevant risk factors on death was verified by receiver operating characteristic (ROC) curve. A total of 59 cases of critically ill neonates were included in this study, with a mortality of 37.3%. The most common primary disease in these cases was neonatal sepsis, followed by neonatal asphyxia, and inborn errors of metabolism (IEM). Univariate analysis showed that the risk factors related to death included primary diseases; the number of organs involved in multiple organ dysfunction syndrome (MODS), neonatal critical illness scores (NCIS), and indications of CRRT; the blood lactate, blood glucose, hemoglobin, and platelet before CRRT initiation; and the incidence of bleeding or thrombosis during CRRT. Multi-factor logistic regression analysis showed that risk factors for death in critically ill neonates receiving CRRT included the occurrence of neonatal sepsis, the number of organs involved in MODS, and the NCIS. ROC curve analysis showed that the number of organs involved in MODS and NCIS had a good predictive value for death in critically ill neonates receiving CRRT, with the areas under the curve (AUC) being 0.700 and 0.810, respectively.
Conclusion: Neonatal sepsis, the number of organs involved in MODS, and NCIS were independent risk factors for death in critically ill neonates receiving CRRT. Moreover, the number of organs involved in MODS and NCIS could effectively predict death in critically ill neonates receiving CRRT.
What is Known: • The population to which CRRT is applicable is gradually expanding from critically ill children to critically ill neonates. • The mortality of critically ill neonates receiving CRRT remains high. | |
What is New: • The most common primary disease in critically ill neonates receiving CRRT was neonatal sepsis, followed by neonatal asphyxia and inborn errors of metabolism (IEM). • The number of organs involved in MODS and NCIS could effectively predict death in critically ill neonates receiving CRRT. |



Similar content being viewed by others
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AKI:
-
Acute kidney injury
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate aminotransferase
- AUC:
-
Areas under the curve
- BUN:
-
Blood urea nitrogen
- CRP:
-
C-reactive protein
- CRRT:
-
Continuous renal replacement therapy
- CVVH:
-
Continuous veno-venous hemofiltration
- CVVHDF:
-
Continuous veno-venous hemodiafiltration
- IEM:
-
Inborn errors of metabolism
- MAP:
-
Mean arterial pressure
- MAS:
-
Meconium aspiration syndrome
- MODS:
-
Multiple organ dysfunction syndrome
- NCIS:
-
Neonatal critical illness score
- NEC:
-
Necrotizing enterocolitis
- NICU:
-
Neonatal intensive care unit
- ROC:
-
Receiver operating characteristic
- SCr:
-
Serum creatinine
- VIS:
-
Vasoactive inotropic score
References
John JC, Taha S, Bunchman TE (2019) Basics of continuous renal replacement therapy in pediatrics. Kidney Res Clin Pract 38:455–461
Raina R, McCulloch M, Nourse P, Sethi SK, Yap HK (2021) Advances in kidney replacement therapy in infants. Adv Chronic Kidney Dis 28:91–104
Cai C, Qiu G, Hong W, Shen Y, Gong X (2020) Clinical effect and safety of continuous renal replacement therapy in the treatment of neonatal sepsis-related acute kidney injury. BMC Nephrol 21:286
Garzotto F, Vidal E, Ricci Z, Paglialonga F, Giordano M, Laforgia N, Peruzzi L, Bellettato M, Murer L, Ronco C (2020) Continuous kidney replacement therapy in critically ill neonates and infants: a retrospective analysis of clinical results with a dedicated device. Pediatr Nephrol 35:1699–1705
Yetimakman AF, Kesici S, Tanyildiz M, Bayrakci US, Bayrakci B (2019) A report of 7-year experience on pediatric continuous renal replacement therapy. J Intensive Care Med 34:985–989
Menon S, Broderick J, Munshi R, Dill L, DePaoli B, Fathallah-Shaykh S, Claes D, Goldstein SL, Askenazi DJ (2019) Kidney support in children using an ultrafiltration device: a multicenter, retrospective study. Clin J Am Soc Nephrol 14:1432–1440
Choi SJ, Ha EJ, Jhang WK, Park SJ (2017) Factors associated with mortality in continuous renal replacement therapy for pediatric patients with acute kidney injury. Pediatr Crit Care Med 18:e56–e61
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC (2016) The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315:801–810
Marshall JC, Deutschman CS (2021) The multiple organ dysfunction syndrome: syndrome, metaphor, and unsolved clinical challenge. Crit Care Med 49:1402–1413
Nada A, Bonachea EM, Askenazi DJ (2017) Acute kidney injury in the fetus and neonate. Semin Fetal Neonatal Med 22:90–97
Sandrio S, Krebs J, Leonardy E, Thiel M, Schoettler JJ (2022) Vasoactive inotropic score as a prognostic factor during (cardio-) respiratory ECMO. J Clin Med 11
Spector BL, Misurac JM (2019) Renal Replacement Therapy in Neonates Neoreviews 20:e697–e710
Sanderson KR, Warady B, Carey W, Tolia V, Boynton MH, Benjamin DK, Jackson W, Laughon M, Clark RH, Greenberg RG (2022) Mortality risk factors among infants receiving dialysis in the neonatal intensive care unit. J Pediatr 242:159–165
Diane Mok TY, Tseng MH, Chiang MC, Lin JL, Chu SM, Hsu JF, Lien R (2018) Renal replacement therapy in the neonatal intensive care unit. Pediatr Neonatol 59:474–480
Dong Y, Basmaci R, Titomanlio L, Sun B, Mercier JC (2020) Neonatal sepsis: within and beyond China. Chin Med J (Engl) 133:2219–2228
Starr MC, Banks R, Reeder RW, Fitzgerald JC, Pollack MM, Meert KL, McQuillen PS, Mourani PM, Chima RS, Sorenson S, Varni JW, Hingorani S, Zimmerman JJ (2020) Severe acute kidney injury is associated with increased risk of death and new morbidity after pediatric septic shock. Pediatr Crit Care Med 21:e686–e695
Coggins SA, Laskin B, Harris MC, Grundmeier RW, Passarella M, McKenna KJ, Srinivasan L (2021) Acute kidney injury associated with late-onset neonatal sepsis: a matched cohort study. J Pediatr 231:185-192.e184
Al-Ayed T, Rahman NU, Alturki A, Aljofan F (2018) Outcome of continuous renal replacement therapy in critically ill children: a retrospective cohort study. Ann Saudi Med 38:260–268
Monard C, Rimmelé T, Ronco C (2019) Extracorporeal blood purification therapies for sepsis. Blood Purif 47(Suppl 3):1–14
Monard C, Abraham P, Schneider A, Rimmelé T (2022) New targets for extracorporeal blood purification therapies in sepsis. Blood Purif 1–7
Sik G, Demirbuga A, Gunhar S, Nisli K, Citak A (2019) Clinical features and indications associated with mortality in continuous renal replacement therapy for pediatric patients. Indian J Pediatr 86:360–364
Badke CM, Mayampurath A, Sanchez-Pinto LN (2022) Multiple organ dysfunction interactions in critically Ill children. Front Pediatr 10:874282
Nishimi S, Sugawara H, Onodera C, Toya Y, Furukawa H, Konishi Y, Sotodate G, Matsumoto A, Ishikawa K, Oyama K (2019) Complications during continuous renal replacement therapy in critically Ill neonates. Blood Purif 47(Suppl 2):74–80
Chen Z, Wang H, Wu Z, Jin M, Chen Y, Li J, Wei Q, Tao S, Zeng Q (2021) Continuous renal-replacement therapy in critically Ill children: practice changes and association with outcome. Pediatr Crit Care Med 22:e605–e612
Dubinsky S, Watt K, Saleeb S, Ahmed B, Carter C, Yeung CHT, Edginton A (2022) Pharmacokinetics of commonly used medications in children receiving continuous renal replacement therapy: a systematic review of current literature. Clin Pharmacokinet 61:189–229
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Funding
This study was funded by the 2020 Shanghai “Science and Technology Innovation Action Plan,” Medical Innovation Research Special Project (20Y11907000).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Jinglin Xu, Xiaoyun Chu, Cheng Cai, and Dongmei Chen. The first draft of the manuscript was written by Jinglin Xu, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Medical Ethics Committee of Quanzhou Maternity and Children’s Hospital and Shanghai Children's Hospital (2020R064-E02), and informed consent was obtained from the patients’ families.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Daniele De Luca
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, J., Chu, X., Zhang, W. et al. Analysis of risk factors for death in 59 cases of critically ill neonates receiving continuous renal replacement therapy: a two-centered retrospective study. Eur J Pediatr 182, 353–361 (2023). https://doi.org/10.1007/s00431-022-04693-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-022-04693-4