Abstract
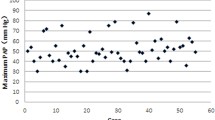
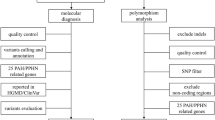
Persistent pulmonary hypertension of the new-borns (PPHN) is one of the main etiologies of morbidity as well as mortality in neonates. Previous studies found that genetic polymorphisms in urea cycle enzymes are associated with PPHN. Few of the genetic polymorphisms in neonates have been recognized with PPHN. We aimed to find out the prevalence of the CPS-I gene polymorphism and to correlate the genotype with the serum nitric oxide (NO) levels in Egyptian neonates with idiopathic PPHN. We included neonates diagnosed with PPH (n = 150) while the control group included healthy neonates with matched age and sex (n = 100). The CPS-I gene polymorphism: A/C, trans-version substitution, rs4399666 genotype was identified using TaqMan-based quantitative PCR. The results revealed that the CPS-I A/C rs4399666 gene polymorphism and lower serum NO levels were significantly associated with idiopathic PPHN in neonates. In addition, serum NO level was significantly associated with an rs4366999 A/C variant gene in idiopathic PPHN (p = 0.001). Univariable regression analysis demonstrated that there was a significant association between CPS-I A/C rs4399666 CC and increased risk of PPHN (odd ratio, 95% CI of 1.8 (0.78 to 1.75), p-value = 0.04).
Conclusion: We concluded that mutant CPS-I A/C rs4399666 minor variant especially the homozygous CC genotype is frequently distributed among the PPHN group. This demonstrates that the presence of mutant CPS-I rs4399666 does not necessarily predispose to the development of PPHN in neonates, but nonetheless, if the C allele is inherited in the homozygous CC genotype, it is associated with a higher risk of PPHN.
What is Known: • Prior studies found that polymorphisms in urea cycle enzyme genes are associated with PPHN. • Association between CPS-1 gene polymorphisms is significantly associated with PPHN. What is New: • The prevalence of CPS-1, A/C trans-version substitution, rs4399666 gene polymorphism in Egyptian neonates presented with idiopathic PPHN. • Mutant CPS-I A/C rs4399666 especially the homozygous CC genotype is more frequently distributed among PPHN, and it is significantly associated with low serum nitric oxide level. |

Similar content being viewed by others
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request. The study data was not presented as an abstract or poster.
Code availability
N/A
Abbreviations
- PPHN:
-
Persistent pulmonary hypertension of the new-borns
- NO:
-
Nitric oxide
- CPS-I:
-
Carbamoyl phosphate synthetase-I
- PVR:
-
Pulmonary vascular resistance
- PAH:
-
Pulmonary arterial hypertension
References
Abman SH (2007) Recent advances in the pathogenesis and treatment of persistent pulmonary hypertension of the newborn. Neonatology 91(4):283–290. https://doi.org/10.1159/000101343
Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, Hanna BD, Rosenzweig EB, Raj JU, Cornfield D, Stenmark KR, Steinhorn R, Thébaud B, Fineman JR, Kuehne T, Feinstein JA, Friedberg MK, Earing M, Barst RJ, Keller RL, Kinsella JP, Mullen M, Deterding R, Kulik T, Mallory G, Humpl T, Wessel DL, American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; and the American Thoracic Society (2015) Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation 132(21):2037–2099. https://doi.org/10.1161/CIR.0000000000000329
Walsh-Sukys MC, Tyson JE, Wright LL, Bauer CR, Korones SB, Stevenson DK, Verter J, Stoll BJ, Lemons JA, Papile LA, Shankaran S, Donovan EF, Oh W, Ehrenkranz RA, Fanaroff AA (2000) Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics 105(1):14–20. https://doi.org/10.1542/peds.105.1.14
Steurer MA, Jelliffe-Pawlowski LL, Baer RJ, Partridge JC, Rogers EE, Keller RL (2017) Persistent pulmonary hypertension of the newborn in late preterm and term infants in California. Pediatrics 139(1):e20161165. https://doi.org/10.1542/peds.2016-1165
Rosenzweig EB et al (2019) Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J 53(1). https://doi.org/10.1183/13993003.01916-2018
Lai M-Y, Chu S-M, Lakshminrusimha S, Lin H-C (2018) Beyond the inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Pediatr Neonatol 59(1):15–23. https://doi.org/10.1016/j.pedneo.2016.09.011
Lipkin PH, Davidson D, Spivak L, Straube R, Rhines J, Chang C (2002) Neurodevelopmental and medical outcomes of persistent pulmonary hypertension in term newborns treated with nitric oxide. J Pediatr 140(3):306–310. https://doi.org/10.1067/mpd.2002.122730
Hilgendorff A, Apitz C, Bonnet D, Hansmann G (2016) Pulmonary hypertension associated with acute or chronic lung diseases in the preterm and term neonate and infant. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart 102(Suppl 2):ii49–ii56. https://doi.org/10.1136/heartjnl-2015-308591
Byers HM, Dagle JM, Klein JM, Ryckman KK, McDonald EL, Murray JC, Borowski KS (2012) Variations in CRHR1 are associated with persistent pulmonary hypertension of the newborn. Pediatr Res 71(2):162–167. https://doi.org/10.1038/pr.2011.24
Catteruccia M, Verrigni D, Martinelli D, Torraco A, Agovino T, Bonafé L, D'Amico A, Donati MA, Adorisio R, Santorelli FM, Carrozzo R, Bertini E, Dionisi-Vici C (2014) Persistent pulmonary arterial hypertension in the newborn (PPHN): a frequent manifestation of TMEM70 defective patients. Mol Genet Metab 111(3):353–359. https://doi.org/10.1016/j.ymgme.2014.01.001
Vonk JM, Postma DS, Maarsingh H, Bruinenberg M, Koppelman GH, Meurs H (2010) Arginase 1 and arginase 2 variations associate with asthma, asthma severity and β2 agonist and steroid response. Pharmacogenet Genomics 20(3):179–186. https://doi.org/10.1097/FPC.0b013e328336c7fd
Pearson DL, Dawling S, Walsh WF, Haines JL, Christman BW, Bazyk A, Scott N, Summar ML (2001) Neonatal pulmonary hypertension: urea-cycle intermediates, nitric oxide production, and carbamoyl-phosphate synthetase function. N Engl J Med 344(24):1832–1838. https://doi.org/10.1056/NEJM200106143442404
Canter JA, Summar ML, Smith HB, Rice GD, Hall LD, Ritchie MD, Motsinger AA, Christian KG, Drinkwater DC Jr, Scholl FG, Dyer KL, Kavanaugh-McHugh AL, Barr FE (2007) Genetic variation in the mitochondrial enzyme carbamyl-phosphate synthetase I predisposes children to increased pulmonary artery pressure following surgical repair of congenital heart defects: a validated genetic association study. Mitochondrion 7(3):204–210. https://doi.org/10.1016/j.mito.2006.11.001
Martinho S, Adão R, Leite-Moreira AF, Brás-Silva C (2020) Persistent pulmonary hypertension of the newborn: pathophysiological mechanisms and novel therapeutic approaches. Front Pediatr 8:342. https://doi.org/10.3389/fped.2020.00342
Trittmann J, Nelin LD, Zmuda EJ, Gastier-Foster JM, Chen B, Backes CH, Frick J, Vaynshtok P, Vieland VJ, Klebanoff MA (2014) Arginase I gene single-nucleotide polymorphism is associated with decreased risk of pulmonary hypertension in bronchopulmonary dysplasia. Acta Paediatr 103(10):e439–e443. https://doi.org/10.1111/apa.12717
Kaluarachchi DC, Smith CJ, Klein JM, Murray JC, Dagle JM, Ryckman KK (2018) Polymorphisms in urea cycle enzyme genes are associated with persistent pulmonary hypertension of the newborn. Pediatr Res 83(1):142–147. https://doi.org/10.1038/pr.2017.143
Muktan D, Singh RR, Bhatta NK, Shah D (2019) Neonatal mortality risk assessment using SNAPPE- II score in a neonatal intensive care unit. BMC Pediatr 19(1):279. https://doi.org/10.1186/s12887-019-1660-y
Shi R, Lewis RS, Panthee DR (2018) Filter paper-based spin column method for cost-efficient DNA or RNA purification. PLoS One 13(12):e0203011. https://doi.org/10.1371/journal.pone.0203011
Kotsopoulos J et al (2009) +331G/A variant in the progesterone receptor gene, postmenopausal hormone use and risk of breast cancer," (in eng). Int J Cancer 125(7):1685–1691. https://doi.org/10.1002/ijc.24477
Faul F, Erdfelder E, Lang A-G, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39(2):175–191. https://doi.org/10.3758/BF03193146
Liu X et al (2019) Identification of genetic factors underlying persistent pulmonary hypertension of newborns in a cohort of Chinese neonates. Respir Res 20(1):174. https://doi.org/10.1186/s12931-019-1148-1
Galambos C et al (2019) Phenotype characterisation of <em>TBX4</em> mutation and deletion carriers with neonatal and pediatric pulmonary hypertension. Eur Respir J:1801965. https://doi.org/10.1183/13993003.01965-2018
Summar ML, Gainer JV, Pretorius M, Malave H, Harris S, Hall LD, Weisberg A, Vaughan DE, Christman BW, Brown NJ (2004) Relationship between carbamoyl-phosphate synthetase genotype and systemic vascular function. Hypertension 43(2):186–191. https://doi.org/10.1161/01.hyp.0000112424.06921.52
Maeda NY, Aiello VD, Santos PC, Thomaz AM, Kajita LJ, Bydlowski SP, Lopes AA (2019) Relation of macrophage migration inhibitory factor to pulmonary hemodynamics and vascular structure and carbamyl-phosphate synthetase i genetic variations in pediatric patients with congenital cardiac shunts. Mediat Inflamm 2019:7305028–7305010. https://doi.org/10.1155/2019/7305028
Moonen RM, Paulussen AD, Souren NY, Kessels AG, Rubio-Gozalbo ME, Villamor E (2007) Carbamoyl phosphate synthetase polymorphisms as a risk factor for necrotizing enterocolitis. Pediatr Res 62(2):188–190. https://doi.org/10.1203/pdr.0b013e3180a0324e
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by AA and DR. The first draft of the manuscript was written by NE and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The case–control study was carried out at the pediatric department after approval from the Local Ethics Committee of Faculty of Medicine, Ain Shams University (FMASU MS 82/2020).
Consent to participate
Written informed consent was obtained from all neonates’ parents. All the methods were conducted following the relevant guidelines, regulations, and Declaration of Helsinki.
Consent for publication
N/A
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Daniele De Luca
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El-Khazragy, N., El Barbary, M., Fouad, H. et al. Association between genetic variations in carbamoyl-phosphate synthetase gene and persistent neonatal pulmonary hypertension. Eur J Pediatr 180, 2831–2838 (2021). https://doi.org/10.1007/s00431-021-04053-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-021-04053-8