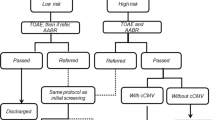
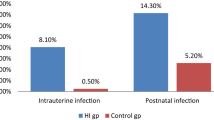
Abstract
The prevalence of permanent childhood hearing impairment (PCHI) in infants admitted to a neonatal intensive care unit (NICU) is higher than that in the general population. Our study objective was to identify risk factors associated with PCHI in infants who required admission to the NICU for more than 48 h. We performed a case–control study, including infants of all gestational ages who were admitted to NICU for more than 48 h and who underwent newborn hearing screening between 2005 and 2019. Infants admitted to NICU and diagnosed with PCHI by formal audiology were classified as “cases”. The “controls” were infants who were admitted to NICU and did not have PCHI. Cases and controls (1:4) were matched based on their birth gestation, birthing place, and treating NICU. The prevalence of PCHI in infants admitted to NICU was 6.3% as compared with our general population prevalence of 0.25%. There were 77 cases and 269 controls during the study period. The median age at diagnosis of PCHI in these infants was 132 days (interquartile range 75.5–518.5). Using regression analysis, “any ventilation episodes”, presence of seizures, and major congenital anomalies were significantly associated with PCHI in infants of all gestational ages. There were higher prevalence of PCHI in preterms (<32 weeks) who received furosemide and lower prevalence with antenatal use of magnesium sulphate.
Conclusions: In our study, the prevalence of hearing loss was high in infants admitted to NICU. Gestation-specific risk factors identified in this case–control study would help in counselling of parents.
What is Known: • In the UK, 1–2/1000 infants are born with hearing loss and infants admitted to the neonatal unit for 48 h or more have increased prevalence of hearing loss (1 in 100 live births). • Identification of risk factors in infants admitted to neonatal unit would help with risk stratification and further management. | |
What is New: • In our study, infants admitted to the neonatal unit had higher prevalence of hearing loss (6.3 in 100 live births). • In infants across all gestational age “any ventilation episodes”, presence of seizures, and severe congenital anomalies were associated with a statistically significant increase in prevalence of hearing loss. Higher prevalence of hearing loss was noted in preterm infants (<32 weeks) who received furosemide treatment and lower prevalence was noted with antenatal use of magnesium sulphate. |

Similar content being viewed by others
Data availability
Original data would be available upon request. Please email your request to pkannanloganathan@nhs.net.
Abbreviations
- AABR:
-
Automated auditory brainstem response
- AOAE:
-
Automated otoacoustic emission
- BPD:
-
Bronchopulmonary dysplasia
- CMV:
-
Cytomegalovirus
- dBnHL:
-
Decibel above normal hearing level
- HIE:
-
Hypoxic ischemic encephalopathy
- IVH:
-
Intraventricular hemorrhage
- MgSO4 :
-
Magnesium sulphate
- NICU:
-
Neonatal intensive care unit
- PDA:
-
Patent ductus arteriosus
- PCHI:
-
Permanent childhood hearing impairment
- PMA:
-
Postmenstrual age
- ROP:
-
Retinopathy of prematurity
References
National Health Services. Newborn hearing screening-your pregnancy and baby guide. Available from: https://www.nhs.uk/conditions/pregnancy-and-baby/newborn-hearing-test/. Accessed 15 June 2020
(2020) https://www.thebsa.org.uk/resources/pure-tone-air-bone-conduction-threshold-audiometry-without-masking/. British Society of Audiology. Available from: https://www.thebsa.org.uk/resources/pure-tone-air-bone-conduction-threshold-audiometry-without-masking/. Accessed 12 Dec 2020
Stadio AD, Molini E, Gambacorta V, Giommetti G, Volpe AD, Ralli M, Lapenna R, Trabalzini F, Ricci G (2019) Sensorineural hearing loss in newborns hospitalized in neonatal intensive care unit: an observational study. Int Tinnitus J 23:31–36
Gabriel MM, Geyer L, McHugh C, Thapa J, Glynn F, Walshe P, Simoes-Franklin C, Viani L (2020) Impact of universal newborn hearing screening on cochlear implanted children in Ireland. Int J Pediatr Otorhinolaryngol 133:109975
Jayagobi PA, Yeoh A, Hee KYM, Sok Bee Lim L, Choo KP, Kun Kiaang HT, Lazaroo D, Daniel LM (2020) Hearing screening outcome in neonatal intensive care unit graduates from a tertiary care centre in Singapore. Child Care Health Dev 46:104–110
Public health England. Guidelines for surveillance and audiological referral for infants and children following newborn hearing screen. Updated 19 July 2019 [cited 2020 15th June]
Ainsworth SB (2014) Neonatal formulary: drug use in pregnancy and the first year of life, 7th ed. John Wiley & Sons, Ltd.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP (2008) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 61:344–349
Eras Z, Konukseven O, Aksoy HT, Canpolat FE, Genç A, Sakrucu ED, Develioğlu O, Dilmen U (2014) Postnatal risk factors associated with hearing loss among high-risk preterm infants: tertiary center results from Turkey. Eur Arch Otorhinolaryngol 271:1485–1490
Khairy MA, Abuelhamed WA, Ahmed RS, El Fouly HES, Elhawary IM (2018) Hearing loss among high-risk newborns admitted to a tertiary neonatal intensive care unit. J Matern Fetal Neonatal Med 31:1756–1761
Blakely T, Pearce N, Lynch J (2019) Case-control studies. Jama 321:806–807
Shepherd E, Salam RA, Middleton P, Makrides M, McIntyre S, Badawi N, Crowther CA (2017) Antenatal and intrapartum interventions for preventing cerebral palsy: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev 8:Cd012077
Kasapoğlu I, Çetinkaya Demir B, Atalay MA et al (2020) Does antenatal magnesium sulphate improve hearing function in premature newborns? J Turk Ger Gynecol Assoc 21(3):187–192
Doyle LW, Crowther CA, Middleton P et al (2009) Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev (1):CD004661. https://doi.org/10.1002/14651858.CD004661.pub3
Borradori C, Fawer CL, Buclin T, Calame A (1997) Risk factors of sensorineural hearing loss in preterm infants. Biol Neonate 71:1–10
El-Barbary MN, Ismail RI, Ibrahim AA (2015) Gentamicin extended interval regimen and ototoxicity in neonates. Int J Pediatr Otorhinolaryngol 79:1294–1298
Schmutzhard J, Glueckert R, Sergi C, Schwentner I, Abraham I, Schrott-Fischer A (2009) Does perinatal asphyxia induce apoptosis in the inner ear? Hear Res 250:1–9
Fitzgerald MP, Reynolds A, Garvey CM, Norman G, King MD, Hayes BC (2019) Hearing impairment and hypoxia ischaemic encephalopathy: incidence and associated factors. Eur J Paediatr Neurol 23:81–86
Bolisetty S, Dhawan A, Abdel-Latif M, Bajuk B, Stack J, Lui K (2014) Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics 133:55–62
Gotardo JW, Volkmer NFV, Stangler GP, Dornelles AD, Bohrer BBA, Carvalho CG (2019) Impact of peri-intraventricular haemorrhage and periventricular leukomalacia in the neurodevelopment of preterms: a systematic review and meta-analysis. PLoS One 14:e0223427
Irony TZ (2018) Case-Control Studies: Using "Real-world" Evidence to Assess Association. Jama 320:1027–1028
Acknowledgements
We would like to thank Kathryn Burn-Thornton, Durham University, for her advice on statistical analysis. We would like to thank Charlotte Kear (paediatric trainee) for her help in data collection. We would like to acknowledge Carol McCormick for her support with manuscript formatting. We would like to acknowledge Professor Win Tin for his support with manuscript appraisal.
Author information
Authors and Affiliations
Contributions
Nair V was responsible for the concept, design, data collection, and interpretation of data, drafted the initial manuscript, and approved the final manuscript. Janakiraman S was responsible for the data collection and interpretation of data and approved the final manuscript. Whittaker S was responsible for the data collection and interpretation of data and approved the final manuscript. Quail J was responsible for the interpretation of data and approved the final manuscript. Foster T was responsible for the interpretation of data and approved the final manuscript. P Loganathan was responsible for the concept, design, data collection, interpretation of data, and data analysis, drafted the initial manuscript, and approved the final manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval
Ethics approval was obtained from the Health Research Authority (HRA ID 287027).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Daniele De Luca
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Health Research Authority approval (HRA ID): 287027
Rights and permissions
About this article
Cite this article
Nair, V., Janakiraman, S., Whittaker, S. et al. Permanent childhood hearing impairment in infants admitted to the neonatal intensive care unit: nested case–control study. Eur J Pediatr 180, 2083–2089 (2021). https://doi.org/10.1007/s00431-021-03983-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-021-03983-7