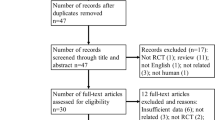
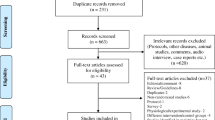
Abstract
The role of macrolides for the prevention and treatment of feeding intolerance (FI) in preterm low birth weight (LBW) infants has not been well established. To assess the efficacy and safety of macrolides to prevent or treat FI in preterm LBW infants. A systematic review and meta-analysis (PROSPERO ID: CRD42020170519) was conducted for English articles published since inception to March 2020, using MEDLINE, EMBASE, and the Cochrane Controlled Trials Register. Search terms included preterm low birth weight infants, macrolides, erythromycin, azithromycin, clarithromycin, and feeding intolerance. Randomized controlled trials (RCTs) assessing the effects of macrolide therapy on the time to achieve full enteral feeding (FEF;150 mL/kg/day), duration of parenteral nutrition (PN), hospitalization, and adverse events in preterm LBW infants were included. Independent extraction of data was done by both authors using predefined data-sheet. Very-low to low-quality evidence from 21 RCTs, 19 for erythromycin (prophylaxis-6, rescue-13) and 2 for clarithromycin (prophylaxis-1, rescue-1) demonstrated a significantly beneficial role of erythromycin for an earlier FEF, both as a prophylaxis (SMD-0.53, 95% CI − 0.74,− 0.33; 6 studies, n = 368) as well as rescue (SMD-1.16, 95% CI − 1.88, − 0.44; 11 studies, n = 664). Rescue therapy was also beneficial for a significant reduction in the duration of PN, hospitalization, incidences of sepsis, necrotizing enterocolitis, and cholestasis. No arrhythmia or infantile hypertrophic pyloric stenosis was reported.
Conclusions: Erythromycin therapy, both as prophylaxis and rescue, is beneficial to reduce the time to achieve FEF in preterm LBW infants, at no higher risk of adverse events.
Trial registration: PROSPERO ID: CRD42020170519







Similar content being viewed by others
References
Brune KD, Donn SM (2018) Enteral Feeding of the Preterm Infant. NeoReviews 19:e645–e653
Ong KK, Kennedy K, Castaneda-Gutierrez E, Forsyth S, Godfrey KM, Koletzko B et al (2015) Postnatal growth in preterm infants and later health outcomes: a systematic review. Acta Paediatr 104:974–986
Chan SH, Johnson MJ, Leaf AA, Vollmer B (2016) Nutrition and neurodevelopmental outcomes in preterm infants: a systematic review. Acta Paediatrica 105:587–599
Kaufman SS, Gondolesi GE, Fishbein TM (2003) Parenteral nutrition associated liver disease. Semin Neonatol 8:375–381
Fanaro S (2013) Feeding intolerance in the preterm infant. Early Hum Dev 89:13–20
Jadcherla SR, Klee G, Berseth CL (1997) Regulation of migrating motor complexes by motilin and pancreatic polypeptide in human infants. Pediatr Res 42:365–369
Jadcherla SR, Kliegman RM (2002) Studies of feeding intolerance in very low birth weight infants: definition and significance. Pediatrics 109:516–517
Oddie SJ, Young L, McGuire W (2017) Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst Rev 8:CD001241
Dorling J, Abbott J, Berrington J, Bosiak B, Bowler U, Boyle E, SIFT Investigators Group et al (2019) Controlled Trial of Two Incremental Milk-Feeding Rates in Preterm Infants. N Engl J Med 381:1434–1443
Karagol BS, Zenciroglu A, Okumus N, Polin RA (2013) Randomized controlled trial of slow vs rapid enteral feeding advancements on the clinical outcomes of preterm infants with birth weight 750-1250 g. J Parenter Enteral Nutr 37:223–228
Nangia S, Bishnoi A, Goel A, Mandal P, Tiwari S, Saili A (2018) Early Total Enteral Feeding in Stable Very Low Birth Weight Infants: A Before and After Study. J Trop Pediatr 64:24–30
Nangia S, Vadivel V, Thukral A, Saili A (2019) Early Total Enteral Feeding versus Conventional Enteral Feeding in Stable Very-Low-Birth-Weight Infants: A Randomised Controlled Trial. Neonatology 115:256–262
Peeters T, Matthijs G, Depoortere I, Cachet T, Hoogmartens J, Vantrappen G (1989) Erythromycin is a motilin receptor agonist. Am J Physiol 257:G470–G474
Costalos C, Gounaris A, Varhalama E, Kokori F, Alexiou N, Kolovou E (2002) Erythromycin as a prokinetic agent in preterm infants. J Pediatr Gastroenterol Nutr 34:23–25
Ng E, Shah VS (2008) Erythromycin for the prevention and treatment of feeding intolerance in preterm infants. Cochrane Database Syst Rev 3:CD001815
Anderson G, Esmonde TS, Coles S, Macklin J, Carnegie C (1991) A comparative safety and efficacy safety of clarithromycin and erythromycin stearate in community acquired pneumonia. J Antimicrob Chemoter 27:117–124
Gokmen T, Ozdemir R, Bozdag S, Oguz SS, Erdeve O, Uras N, Dilmen U (2013) Clarithromycin treatment in preterm infants: a pilot study for prevention of feeding intolerance. J Matern Fetal Neonatal Med 26:1528–1531
Moshiree B, McDonald R, Hou W, Toskes PP (2010) Comparison of the effect of azithromycin versus erythromycin on antroduodenal pressure profiles of patients with chronic functional gastrointestinal pain and gastroparesis. Dig Dis Sci 55:675–683
Cooper WO, Griffin MR, Arbogast P, Hickson GB, Gautam S, Ray WA (2002) Very early exposure to erythromycin and infantile hypertrophic pyloric stenosis. Arch Pediatr Adolesc Med 156:647–650
Maheshwai N (2007) Are young infants treated with erythromycin at risk for developing hypertrophic pyloric stenosis? Arch Dis Child 92:271–273
Ludvigsson JF, Lundholm C, Örtqvist AK, Almqvist C (2016) No association between macrolide treatment in infancy and later pyloric stenosis in Sweden. Eur J Epidemiol 31:331–332
Murchison L, De Coppi P, Eaton S (2016) Post-natal erythromycin exposure and risk of infantile hypertrophic pyloric stenosis: a systematic review and meta-analysis. Pediatr Surg Int 32:1147–1152
Benoit A, Bodiou C, Villain E, Bavoux F, Checoury A, Badoual J (1991) QT prolongation and circulatory arrest after an injection of erythromycin in a newborn infant. Arch Fr Pediatr 48:39–41
Katapadi K, Kostandy G, Katapadi M, Hussain KM, Schifter D (1997) A review of erythromycin-induced malignant tachyarrhythmia—torsade de pointes. A case report. Angiology 48:821–826
Lakritz J, Wilson DW (1997) Erythromycin: pharmacokinetics, bioavailability, antimicrobial activity, and possible mechanisms associated with adverse reactions. Proceedings of the Annual Convention of the AAEP 43:83–86
Buck ML (2010) Erythromycin as a gastrointestinal prokinetic agent in infants. Pediatr Pharmacother 16:1–4
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ 339:b2535
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (2019) editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.0. Chichester (UK): John Wiley & Sons
Borenstein M, Hedges L, Higgins J, Rothstein H (2013) Comprehensive Meta-Analysis Version 3. Biostat, Englewood
Review Manager (RevMan) [Computer program] (2020) Version 5.4, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14:135
Schünemann H, Brożek J, Guyatt G, Oxman A (2013) editors. GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group. Available from: guidelinedevelopment.org/handbook
GRADEpro GDT: GRADEpro Guideline Development Tool [Software] (2020) McMaster University (developed by Evidence Prime, Inc.). Available from: gradepro.org
Oei J, Lui K (2001) A placebo-controlled trial of low-dose erythromycin to promote feed tolerance in preterm infants. Acta Paediatr 90:904–908
Mohammadizadeh M, Ghazinour M, Iranpour R (2010) Efficacy of prophylactic oral erythromycin to improve enteral feeding tolerance in preterm infants: a randomised controlled study. Singapore Med J 51:952–956
Stenson BJ, Middlemist L, Lyon AJ (1998) Influence of erythromycin on establishment of feeding in preterm infants: observations from a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 79:F212–F214
Patole SK, Almonte R, Kadalraja R, Tuladhar R, Muller R, Whitehall JS (2000) Can prophylactic oral erythromycin reduce time to full enteral feeds in preterm neonates? Int J Clin Pract 54:504–508
Gokmen T, Oguz S, Bozdag S, Erdeve O, Uras N, Dilmen U (2012) A controlled trial of erythromycin and UDCA in premature infants during parenteral nutrition in minimizing feeding intolerance and liver function abnormalities. J Perinatol 32:123–128
Sukmawati M, Rohsiswatmo R, Suradi R, Gayatri P (2017) Efficacy of oral erythromycin to enhance feeding tolerance in preterm infants. Paediatr Indones 57:154–159
Cairns PA, Craig S, Tubman R, Roberts RS, Wilson J, Schmidt B (2002) Randomised controlled trial of low-dose erythromycin in preterm infants with feed intolerance. Pediatr Res 51:379A
ElHennawy AA, Sparks JW, Armentrout D, Huseby V, Berseth CL (2003) Erythromycin fails to improve feeding outcome in feeding-intolerant preterm infants. J Pediatr Gastroenterol Nutr 37:281–286
Aly H, Abdel-Hady H, Khashaba M, El-Badry N (2007) Erythromycin and feeding intolerance in premature infants: a randomized trial. J Perinatol 27:39–43
Ng PC, So KW, Fung KSC, Lee CH, Fok TF, Wong E, Wong W, Cheung KL, Cheng AF (2001) Randomised controlled study of oral erythromycin for treatment of gastrointestinal dysmotility in preterm infants. Arch Dis Child Fetal Neonatal Ed 84:F177–F182
Ng SC, Gomez JM, Rajadurai VS, Saw S, Quak S (2003) Establishing enteral feeding in preterm infants with feeding intolerance; A randomized controlled study of low-dose erythromycin. J Pediatr Gastroenterol Nutr 37:554–548
Madani A, Pishva N, Pourarian SH, Zarkesh M (2004) The efficacy of oral erythromycin in enhancement of milk tolerance in premature infants: A randomized controlled trial. Iran J Med Sci 29:1–4
Nuntnarumit P, Kiatchoosakun P, Tantiprapa W, Boonkasidecha S (2006) Efficacy of oral erythromycin for treatment of feeding intolerance in preterm infants. J Pediatr 148:600–605
Ng PC, Lee CH, Wong SP, Lam HS, Liu FY, So KW et al (2007) High-dose oral erythromycin decreased the incidence of parenteral nutrition-associated cholestasis in preterm infants. Gastroenterology 132:1726–1739
Zarkesh M, Haryalch K, Madani A, Pishva N, Poorarian S (2010) Efficacy of high-dose oral erythromycin on enhancement of feeding tolerance in premature neonates. Iran J Neonatol 1:15–19
Mansi Y, Abdelaziz N, Ezzeldin Z, Ibrahim R (2011) Randomized Controlled Trial of a High Dose of Oral Erythromycin for the Treatment of Feeding Intolerance in Preterm Infants. Neonatology 100:290–294
Ng YY, Su PH, Chen JY, Quek YW, Hu JM, Lee IC, Lee HS, Chang HP (2012) Efficacy of intermediate-dose oral erythromycin on very low birth weight infants with feeding intolerance. Pediatr Neonatol 53:34–40
Saboute M, Mazouri A, NaimiDehnavi F, Khalesi N, Farahani Z (2018) Influence of high-dose oral erythromycin on feeding intolerance in preterm neonates: A randomized controlled trial. Med J Islam Repub Iran 32:9
Armanian A, Mousavi A, Mohammadizadeh M, Salehimehr N, Hassanzade A (2019) Evaluating the Effect of the Intermediate-Dose Oral Erythromycin on the Treatment of Feeding Intolerance in Premature Neonates: A Randomized Clinical Trial. Iran Red Crescent Med J 21:e92790
Berseth CL (1996) Gastrointestinal motility in the neonate. Clin Perinatol 23:179–190
Takanashi H, Cynshi O (2009) Motilides: a long and winding road: lessons from mitemcinal (GM-611) on diabetic gastroparesis. Regul Pept 155:18–23
Jadcherla SR, Berseth CL (2002) Effect of erythromycin on gastroduodenal contractile activity in developing neonates. J Pediatr Gastroenterol Nutr 34:16–22
Bruley des Varannes S, Parys V, Ropert A, Chayvialle JA, Rozé C, Galmiche JP (1995) Erythromycin enhances fasting and postprandial proximal gastric tone in humans. Gastroenterology 109:32–39
Janssens J, Peeters TL, Vantrappen G, Tack J, Urbain JL, De Roo M et al (1990) Improvement of gastric emptying in diabetic gastroparesis by erythromycin. Preliminary studies. N Engl J Med 322:1028–1031
Mathis C, Malbert CH (1998) Changes in pyloric resistance induced by erythromycin. Neurogastroenterol Motil 10:131–138
Banerjee A, Chitnis UB, Jadhav SL, Bhawalkar JS, Chaudhury S (2009) Hypothesis testing, type I and type II errors. Ind Psychiatry J 18:127–131
Boyd CA, Quigley MA, Brocklehurst P (2007) Donor breast milk versus infant formula for preterm infants: systematic review and metaanalysis. Arch Dis Child Fetal Neonatal Ed 92:F169–F175
Quigley M, McGuire W (2014) Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev 22:CD002971
Sisk PM, Lambeth TM, Rojas MA, Lightbourne T, Barahona M, Anthony E, Auringer ST (2017) Necrotizing Enterocolitis and growth in preterm infants fed predominantly maternal milk, pasteurized donor milk, or preterm formula: a retrospective study. Am J Perinatol 34:676–683
Corpeleijn WE, de Waard M, Christmann V, van Goudoever JB, Jansen-van der Weide MC, Kooi EM et al (2016) Effect of Donor Milk on Severe Infections and Mortality in Very Low-Birth-Weight Infants: The Early Nutrition Study Randomized Clinical Trial. JAMA Pediatr 170:654–661
Costa S, Maggio L, Alighieri G, Barone G, Cota F, Vento G (2018) Tolerance of preterm formula versus pasteurized donor human milk in very preterm infants: a randomized non-inferiority trial. Ital J Pediatr 44:96
Gouyon JB, Benoit A, Bétremieux P, Sandre D, Sgro C, Bavoux F, Beneton C, Badoual J (1994) Cardiac toxicity of intravenous erythromycin lactobionate in preterm infants. Pediatr Infect Dis J 13:840–841
Sims PJ, Waites KB, Crouse DT (1994) Erythromycin lactobionate toxicity in preterm neonates. Pediatr Infect Dis J 13:164–167
Otterson MF, Sarna SK (1990) Gastrointestinal motor effects of erythromycin. Am J Physiol 259:G355–G363
Coulie B, Tack J, Peeters T, Janssens J (1998) Involvement of two different pathways in the motor effects of erythromycin on the gastric antrum in humans. Gut 43:395–400
Berseth CL (1990) Neonatal small intestinal motility: motor responses to feeding in term and preterm infants. J Pediatr 117:777–782
Bisset WM, Watt JB, Rivers RP, Milla PJ (1988) Ontogeny of fasting small intestinal motor activity in the human infant. Gut 29:483–488
Curry JI, Lander TD, Stringer MD (2001) Erythromycin as a prokinetic agent in infants and children. Aliment Pharmacol Ther 15:595–603
Westphal JF, Vetter D, Brogard JM (1994) Hepatic side effects of antibiotics. J Antimicrob Chemother 33:387–401
Chi C, Buys N, Li C, Sun J, Yin C (2019) Effects of Prebiotics on Sepsis, Necrotizing Enterocolitis, Mortality, Feeding Intolerance, Time to Full Enteral Feeding, Length of Hospital Stay, and Stool Frequency in Preterm Infants: A Meta-Analysis. Eur J Clin Nutr 73:657–670
Indrio F, Riezzo G, Tafuri S, Ficarella M, Carlucci B, Bisceglia M, Polimeno L, Francavilla R (2017) Probiotic Supplementation in Preterm: Feeding Intolerance and Hospital Cost. Nutrients 9:965
Cryan JF, O’Mahony SM (2011) The microbiome-gut-brain axis: From bowel to behavior. Neurogastroenterol Motil 23:187–192
Athalye-Jape G, Patole S (2019) Probiotics for preterm infants - time to end all controversies. Microb Biotechnol 12:249–253
Author information
Authors and Affiliations
Contributions
Sriparna Basu (SB) and Susan Smith (SS) conceptualized and planned this systematic review. SB searched the literature, collected the study details and outcome data using a predetermined form designed for this purpose in consultation with SS. The disagreement was resolved by discussion. The risk of bias for each study was assessed by SB. Both the authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Daniele De Luca
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Web Figure 1
Forest plots for the days to achieve full enteral feeding (150 mL/kg/day): A Low-dose erythromycin prophylaxis, B High-dose erythromycin prophylaxis (PNG 54 kb)
Web Figure 2
Forest plots for the days to achieve full enteral feeding (150 mL/kg/day): A Low-dose erythromycin rescue, B High-dose erythromycin rescue (PNG 378 kb)
Web Figure 3
Forest plots for the days to achieve full enteral feeding (150 mL/kg/day) after erythromycin rescue therapy: A Infants of gestational age ≥ 32 weeks B Infants of gestational age < 32 weeks (PNG 342 kb)
Web Figure 4
Forest plots for the duration of hospitalization after erythromycin rescue therapy: A Infants of gestational age ≥ 32 weeks B Infants of gestational age < 32 weeks (PNG 292 kb)
Web Table 1
Details of database search (DOCX 13 kb)
Web Table 2
Details of excluded articles (DOCX 21 kb)
Web Table 3
Meta-regression of individual outcome on mean gestation for studies using erythromycin as prophylaxis (DOCX 13 kb)
Web Table 4
Meta-regression of individual outcome on mean gestation for studies using erythromycin as treatment (DOCX 14 kb)
Web Table 5
Publication bias for individual outcome (Egger’s regression) for studies using erythromycin as prophylaxis (DOCX 13 kb)
Web Table 6
Publication bias for individual outcome (Egger’s regression) for studies using erythromycin as treatment (DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Basu, S., Smith, S. Macrolides for the prevention and treatment of feeding intolerance in preterm low birth weight infants: a systematic review and meta-analysis. Eur J Pediatr 180, 353–378 (2021). https://doi.org/10.1007/s00431-020-03814-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-020-03814-1