Abstract
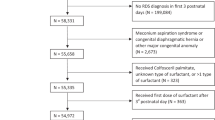
The guidelines for surfactant therapy are largely based on studies done in developed coun1tries wherein the facility infrastructure, patient profile, and clinical practices are different from low- and middle-income countries (LMICs). Though SRT is widely practiced in developing countries, there exists variability in clinical practice. Our objective was to identify the factors which would predict the need of surfactant administration and develop a “clinical respiratory distress (RD) score” for surfactant administration in preterm neonates with respiratory distress. A prospective observational study was conducted in 153 preterm infants (260/7 to 346/7 weeks gestation) with respiratory distress who were managed with CPAP and/or surfactant where indicated. Gestation < 32 weeks, no antenatal corticosteroid (ANS), hypothermia at admission, Apgar score < 3 at 1 minute, and Silverman score > 2 at 2 hours were found to be the significant factors in predicting surfactant requirement in multivariate regression analysis. A seven point scale was developed and categorized into two categories as < 4 and ≥ 4. The sensitivity, specificity, PPV, and NPV were 67%, 87%, 86%, and 68%, respectively, with a cutoff score ≥ 4. The positive likelihood ratio was 5.07 (95% CI 2.71–9.48), and negative likelihood ratio was 0.38 (95% CI 0.28–0.52). The observed rate of surfactant administration was found to be around 32% when the composite score was below four, and the rate increased to almost 86% when the composite score was ≥ 4. The predictive accuracy of the model was subsequently evaluated in a cohort of 56 preterm infants with respiratory distress.. Sensitivity, specificity and positive and negative predictive value during the validation phase were 97%, 73%, 85%, and 94%, respectively. With a composite score less than 4, the observed rate of surfactant administration was 6% (95% CI 1%–28%) as against the model predicted rate of 24%, while with composite score ≥ 4, the observed rate was 85% (95% CI 69%–94%) as against the model predicted rate of 90%.
Conclusion: “Clinical RD score” is a simple score, which can be utilized for decision-making for early surfactant administration for preterm infants (260/7 to 346/7 weeks gestation) with respiratory distress.
Trial Registration: NCT03273764
What is Known: • Both CPAP and surfactant therapy are effective in management of preterm infants with RDS. • The efficacy of surfactant replacement therapy is better when it is administered early in the course of disease. | |
What is New: • Many of the known risk factors for RDS do not predict surfactant requirement. • “Composite RD score” comprising of five independent predictors of surfactant requirement with a numeric cutoff may help decide which preterm neonates with respiratory distress need early surfactant administration in low- and middle-income countries. |


Similar content being viewed by others
Abbreviations
- ANS:
-
antenatal steroid
- CI:
-
confidence interval
- CPAP:
-
continuous positive airway pressure
- RD:
-
respiratory distress
- HMD:
-
hyaline membrane disease
- LMIC:
-
low- and middle-income country
- LSCS:
-
lower segment caesarean section
- PIH:
-
pregnancy induced hypertension
- ROC:
-
receiver operator characteristics
- SRT:
-
surfactant replacement therapy
References
Lawn JE, Blencowe H, Oza S, You D, Lee AC, Waiswa P et al (2014) Lancet every newborn study group. Every newborn: progress, priorities, and potential beyond survival. Lancet 384(9938):189–205
Wambach JA, Hamvas A (2014) Respiratory distress syndrome in the neonate. In: Martin RJ, Fanaroff AA, Walsh MC (eds) Fanaroff and Martin’s neonatal-perinatal medicine diseases of the fetus and infant, 10th edn. Mosby Elsevier, Philadelphia, pp 1074–1086
Gregory GA, Kitterman JA, Phibbs RH, Tooley WH, Hamilton WK (1971) Treatment of the idiopathic respiratory-distress syndrome with continuous positive airway pressure. N Engl J Med 284(24):1333–1340
Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB, COIN Trial Investigators (2008) Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med 358(7):700–708
Thukral A, Sankar MJ, Chandrasekaran A, Agarwal R, Paul VK (2016) Efficacy and safety of CPAP in low- and middle-income countries. J Perinatol 36(Suppl 1):S21–S28
Halliday HL (2008) Surfactants: past, present and future. J Perinatol 28(Suppl 1):S47–S56
Rojas-Reyes MX, Morley CJ, Soll R (2012) Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev 3:CD000510
Bahadue FL, Soll R (2012) Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database Syst Rev 11:CD001456
Polin RA, Carlo WA (2014) Committee on Fetus and Newborn; American Academy of Pediatrics. Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics 133(1):156–163
Stevens TP, Harrington EW, Blennow M, Soll RF (2007) Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst Rev 4:CD003063
Report of National Neonatal perinatal Database (NNPD) 2002-2003. Available from http://www.newbornwhocc.org/nnpo_html. Accessed Dec 2014
Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R (1991) New Ballard Score, expanded to include extremely premature infants. J Pediatr 119(3):417–423
Silverman WA, Andersen DH (1956) A controlled clinical trial of effects of water mist on obstructive respiratory signs, death rate and necropsy findings among premature infants. Pediatrics 17(1):1–10
Fenton TR, Kim JH (2013) A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 13:59
Hosmer D, Lemeshow S (2010) Applied logistic regression. Wiley, New York
Egan J (1975) Signal detection theory and ROC analysis. Academic, New York
Stata: software for statistics and data science [Internet]. Stata.com. 2019. Available from: https://www.stata.com. Accessed Oct 2016
Sullivan LM, Massaro JM, D'Agostino RB Sr (2004) Presentation of multivariate data for clinical use: the Framingham study risk score functions. Stat Med 23(10):1631–1660
Verder H, Albertsen P, Ebbesen F, Greisen G, Robertson B, Bertelsen A, Agertoft L, Djernes B, Nathan E, Reinholdt J (1999) Nasal continuous positive airway pressure and early surfactant therapy for respiratory distress syndrome in newborns of less than 30 weeks' gestation. Pediatrics 103(2):E24
Reininger A, Khalak R, Kendig JW, Ryan RM, Stevens TP, Reubens L, D'Angio CT (2005) Surfactant administration by transient intubation in infants 29 to 35 weeks' gestation with respiratory distress syndrome decreases the likelihood of later mechanical ventilation: a randomized controlled trial. J Perinatol 25(11):703–708
Rojas MA, Lozano JM, Rojas MX, Laughon M, Bose CL, Rondon MA (2009) et al; Colombian Neonatal Research Network. Very early surfactant without mandatory ventilation in premature infants treated with early continuous positive airway pressure: a randomized, controlled trial. Pediatrics 123(1):137–142
Kandraju H, Murki S, Subramanian S, Gaddam P, Deorari A, Kumar P (2013) Early routine versus late selective surfactant in preterm neonates with respiratory distress syndrome on nasal continuous positive airway pressure: a randomized controlled trial. Neonatology 103(2):148–154
Narang A, Kumar P, Dutta S, Kumar R (2001) Surfactant therapy for hyaline membrane disease: the Chandigarh experience. Indian Pediatr 38(6):640–646
Sandri F, Plavka R, Ancora G, Simeoni U, Stranak Z, Martinelli S, CURPAP Study Group et al (2010) Prophylactic or early selective surfactant combined with nCPAP in very preterm infants. Pediatrics 125(6):e1402–e1409
SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network, Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, Laptook AR et al (2010) Early CPAP versus surfactant in extremely preterm infants. N Engl J Med 362(21):1970–1979
Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, Saugstad OD, Simeoni U, Speer CP, Vento M, Visser GH, Halliday HL (2017) European consensus guidelines on the management of respiratory distress syndrome - 2016 Update. Neonatology 111(2):107–125
Agertoft L, Djernes B, Nathan E, Reinholdt J, Dargaville PA, Aiyappan A et al (2013) Continuous positive airway pressure failure in preterm infants: incidence, predictors and consequences. Neonatology 104:8–14
Sweet D, Carnielli V, Greisen G, Hallman M, Ozek E, te Pas A, Plavka R, Roehr CC, Saugstad OD, Simeoni U, Speer CP, Vento M, Visser GHA, Halliday HL (2019) European consensus guidelines on the management of respiratory distress syndrome – 2019 Update. Neonatology 115(4):432–450
Author information
Authors and Affiliations
Contributions
SN conceptualized and designed the study, supervised the collection of and analysis of data, and critically revised and finalized the manuscript; AT participated in the study design and critical revision of the manuscript and helped in statistical analysis; DN participated in the study design, recruited patients, collected data, and drafted the manuscript; CP has done statistical analysis; and all authors approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflicts of interest.
Ethical approval
The study protocol was approved by the institution’s ethics committee. Ethical approval for all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statement of informed consent
Written informed consent was obtained from the parents of participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nanda, D., Nangia, S., Thukral, A. et al. A new clinical respiratory distress score for surfactant therapy in preterm infants with respiratory distress. Eur J Pediatr 179, 603–610 (2020). https://doi.org/10.1007/s00431-019-03530-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-019-03530-5