Abstract
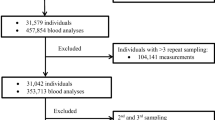
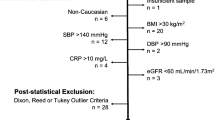
The aim was to elude differences in published paediatric reference intervals (RIs) and the implementations hereof in terms of classification of samples. Predicaments associated with transferring RIs published elsewhere are addressed. A local paediatric (aged 0 days to < 18 years) population of platelet count, haemoglobin level and white blood cell count, based on first draw samples from general practitioners was established. PubMed was used to identify studies with transferable RIs. The classification of local samples by the individual RIs was evaluated. Transference was done in accordance with the Clinical and Laboratory Standards Institute EP28-A3C guideline. Validation of transference was done using a quality demand based on biological variance. Twelve studies with a combined 28 RIs were transferred onto the local population, which was derived from 20,597 children. Studies varied considerably in methodology and results. In terms of classification, up to 63% of the samples would change classification from normal to diseased, depending on which RI was applied. When validating the transferred RIs, one RI was implementable in the local population. Conclusion: Published paediatric RIs are heterogeneous, making assessment of transferability problematic and resulting in marked differences in classification of paediatric samples, thereby potentially affecting diagnosis and treatment of children.
What is Known: • Reference intervals (RIs) are fundamental for the interpretation of paediatric samples and thus correct diagnosis and treatment of the individual child. • Guidelines for the establishment of adult RIs exist, but there are no specific recommendations for establishing paediatric RIs, which is problematic, and laboratories often implement RIs published elsewhere as a consequence. | |
What is New: • Paediatric RIs published in peer-reviewed scientific journals differ considerably in methodology applied for the establishment of the RI. • The RIs show marked divergence in the classification of local samples from healthy children. |


Similar content being viewed by others
Abbreviations
- RI:
-
Reference interval
- plt:
-
Platelet count
- hgb:
-
Haemoglobin level
- WBC:
-
White blood cell
- GP:
-
General practitioner
- CLSI:
-
Clinical and Laboratory Standards Institute
- QD:
-
Quality demand
- IFCC:
-
Federation of Clinical Chemistry and Laboratory Medicine
- CBC:
-
Complete blood count
- LH:
-
Lillebaelt Hospital
- OUH:
-
Odense University Hospital
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- ER:
-
Emergency room
References
Adeli K, Raizman JE, Chen Y, Higgins V, Nieuwesteeg M, Abdelhaleem M, Wong SL, Blais D (2015) Complex biological profile of hematologic markers across pediatric, adult, and geriatric ages: establishment of robust pediatric and adult reference intervals on the basis of the Canadian health measures survey. Clin Chem 61(8):1075–1086. https://doi.org/10.1373/clinchem.2015.240531
Aldrimer M, Ridefelt P, Rodoo P, Niklasson F, Gustafsson J, Hellberg D (2013) Population-based pediatric reference intervals for hematology, iron and transferrin. Scand J Clin Lab Invest 73(3):253–261. https://doi.org/10.3109/00365513.2013.769625
Biino G, Santimone I, Minelli C, Sorice R, Frongia B, Traglia M, Ulivi S, Di Castelnuovo A, Gogele M, Nutile T, Francavilla M, Sala C, Pirastu N, Cerletti C, Iacoviello L, Gasparini P, Toniolo D, Ciullo M, Pramstaller P, Pirastu M, de Gaetano G, Balduini CL (2013) Age- and sex-related variations in platelet count in Italy: a proposal of reference ranges based on 40987 subjects' data. PLoS One 8(1):e54289. https://doi.org/10.1371/journal.pone.0054289
Carraro P, Vettore G, Padoan A, Piva E, Plebani M (2015) Complete blood count at the ED: preanalytic variables for hemoglobin and leukocytes. Am J Emerg Med 33(9):1152–1157. https://doi.org/10.1016/j.ajem.2015.05.011
CLSI (2010) Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline - third edition. CLSI document EP28-A3C, vol Third edition. Clinical and Laboratory Standards Institute, Wayne
Colantonio DA, Kyriakopoulou L, Chan MK, Daly CH, Brinc D, Venner AA, Pasic MD, Armbruster D, Adeli K (2012) Closing the gaps in pediatric laboratory reference intervals: a CALIPER database of 40 biochemical markers in a healthy and multiethnic population of children. Clin Chem 58(5):854–868. https://doi.org/10.1373/clinchem.2011.177741
Daae LN, Hallerud M, Halvorsen S (1991) A comparison between haematological parameters in ‘capillary’ and venous blood samples from hospitalized children aged 3 months to 14 years. Scand J Clin Lab Invest 51(7):651–654
Dortschy R, Rosario AS, Scheidt-Nave C, Thierfelder W, Thamm M, Gutsche J, Markert A (2009) Bevölkerungsbezogene Verteilungswerte ausgewählter Laborparameter aus der Studie zur Gesundheit von Kindern und Jugendlichen in Deutschland (KiGGS), vol 2017, Berlin
Favaloro EJ, Lippi G (2017) Translational aspects of developmental hemostasis: infants and children are not miniature adults and even adults may be different. Ann Transl Med 5(10):212. https://doi.org/10.21037/atm.2017.04.18
Feusner JH, Behrens JA, Detter JC, Cullen TC (1979) Platelet counts in capillary blood. Am J Clin Pathol 72(3):410–414
Fraser CG, Hyltoft Petersen P, Libeer JC, Ricos C (1997) Proposals for setting generally applicable quality goals solely based on biology. Ann Clin Biochem 34(Pt 1):8–12. https://doi.org/10.1177/000456329703400103
Giacomini A, Legovini P, Gessoni G, Antico F, Valverde S, Salvadego MM, Manoni F (2001) Platelet count and parameters determined by the Bayer ADVIA 120 in reference subjects and patients. Clin Lab Haematol 23(3):181–186
Hinchliffe RF, Bellamy GJ, Bell F, Finn A, Vora AJ, Lennard L (2013) Reference intervals for red cell variables and platelet counts in infants at 2, 5 and 13 months of age: a cohort study. J Clin Pathol 66(11):962–966. https://doi.org/10.1136/jclinpath-2013-201742
Hollowell JG, van Assendelft OW, Gunter EW, Lewis BG, Najjar M, Pfeiffer C, Centers for Disease C, Prevention NCfHS (2005) Hematological and iron-related analytes--reference data for persons aged 1 year and over: United States, 1988–94. Vital Health Stat 11(247):1–156
Kayiran SM, Ozbek N, Turan M, Gurakan B (2003) Significant differences between capillary and venous complete blood counts in the neonatal period. Clin Lab Haematol 25(1):9–16
Krleza JL, Dorotic A, Grzunov A, Maradin M, Croatian Society of Medical B, Laboratory M (2015) Capillary blood sampling: national recommendations on behalf of the Croatian Society of Medical Biochemistry and Laboratory Medicine. Biochem Med (Zagreb) 25(3):335–358. https://doi.org/10.11613/BM.2015.034
Lykkeboe S, Nielsen CG, Christensen PA (2018) Indirect method for validating transference of reference intervals. Clin Chem Lab Med 56(3):463–470. https://doi.org/10.1515/cclm-2017-0574
Monagle P, Barnes C, Ignjatovic V, Furmedge J, Newall F, Chan A, De Rosa L, Hamilton S, Ragg P, Robinson S, Auldist A, Crock C, Roy N, Rowlands S (2006) Developmental haemostasis. Impact for clinical haemostasis laboratories. Thromb Haemost 95(2):362–372. https://doi.org/10.1160/TH05-01-0047
Ozbek N, Gurakan B, Kayiran SM (2000) Complete blood cell counts in capillary and venous blood of healthy term newborns. Acta Haematol 103(4):226–228 doi:41056
Ridefelt P, Hellberg D, Aldrimer M, Gustafsson J (2014) Estimating reliable paediatric reference intervals in clinical chemistry and haematology. Acta Paediatr 103(1):10–15. https://doi.org/10.1111/apa.12438
Romeo J, Warnberg J, Gomez-Martinez S, Diaz LE, Moreno LA, Castillo MJ, Redondo C, Baraza JC, Sola R, Zamora S, Marcos A, group A (2009) Haematological reference values in Spanish adolescents: the AVENA study. Eur J Haematol 83(6):586–594. https://doi.org/10.1111/j.1600-0609.2009.01326.x
Sankaran VG, Orkin SH (2013) The switch from fetal to adult hemoglobin. Cold Spring Harb Perspect Med 3(1):a011643. https://doi.org/10.1101/cshperspect.a011643
Steven J, Soldin CB, Wong EC (2011) Pediatric reference ranges, 7th edn. AACC Press, Washington, DC
Stirnadel-Farrant HA, Galwey N, Bains C, Yancey C, Hunt CM (2015) Children’s liver chemistries vary with age and gender and require customized pediatric reference ranges. Regul Toxicol Pharmacol 73(1):349–355. https://doi.org/10.1016/j.yrtph.2015.07.013
Tahmasebi H, Higgins V, Fung AWS, Truong D, White-Al Habeeb NMA, Adeli K (2017) Pediatric reference intervals for biochemical markers: gaps and challenges, recent national initiatives and future perspectives. EJIFCC 28(1):43–63
Tai DY, Chan KW, Chee YC, Mak KH (1995) Comparison of platelet counts in simultaneous venous and capillary blood samples using an automated platelet analyser. Singap Med J 36(3):263–266
Taylor MR, Holland CV, Spencer R, Jackson JF, O'Connor GI, O'Donnell JR (1997) Haematological reference ranges for schoolchildren. Clin Lab Haematol 19(1):1–15
Tukey JW (1977) Exploratory data analysis. Addison-Wesley, Reading
Unger G, Benozzi SF, Campion A, Pennacchiotti GL (2018) Preanalytical phase: effects of water ingestion during fasting on routine hematological parameters in a small cohort of young women. Clin Chim Acta 483:126–129. https://doi.org/10.1016/j.cca.2018.04.019
van Assendelft OW (1987) The international system of units (SI) in historical perspective. Am J Public Health 77(11):1400–1403
World HO (2016) Under-five mortality rate (per 1000 live births). http://apps.who.int/gho/data/view.xgswcah.10-viz. Accessed Accessed June 2018 June 2018
World HO (2018) Density of physicians (total number per 1000 population, latest available year). WHO. http://www.who.int/gho/health_workforce/physicians_density/en/. Accessed Accessed June 2018 2018
Zierk J, Arzideh F, Haeckel R, Rascher W, Rauh M, Metzler M (2013) Indirect determination of pediatric blood count reference intervals. Clin Chem Lab Med 51(4):863–872. https://doi.org/10.1515/cclm-2012-0684
Zierk J, Arzideh F, Rechenauer T, Haeckel R, Rascher W, Metzler M, Rauh M (2015) Age- and sex-specific dynamics in 22 hematologic and biochemical analytes from birth to adolescence. Clin Chem 61(7):964–973. https://doi.org/10.1373/clinchem.2015.239731
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to the present study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by Peter de Winter
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alnor, A.B., Vinholt, P.J. Paediatric reference intervals are heterogeneous and differ considerably in the classification of healthy paediatric blood samples. Eur J Pediatr 178, 963–971 (2019). https://doi.org/10.1007/s00431-019-03377-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-019-03377-w