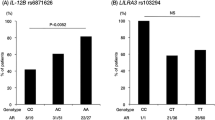
Abstract
Kawasaki disease (KD) is a systemic vasculitis childhood disease frequently complicating coronary artery lesions (CALs). Recently, the gene encoding a disintegrin and metalloprotease 17 (ADAM17) was found to modify vascular pathology in humans by differentially regulating the transforming growth factor-β (TGF-β) signaling pathway, which affects KD/CAL susceptibility. To explore the potential role of ADAM17 in KD occurrence and outcomes, we investigated the association of 28 single nucleotide polymorphisms (SNPs) in ADAM17 and three pathway genes of TGF-β signaling with KD phenotypes in a Han Chinese population, including 392 KD patients and 421 non-KD controls. Three ADAM17 SNPs showed an association with KD risk, which was further confirmed by haplotype analysis. The effect of ADAM17 on KD was also shown by multi-variable logistic regression analysis. In two-locus model analyses with SNPs in ADAM17 and TGF-β signaling pathway genes, stronger compound effects on the risk of KD and secondary CAL formation were observed relative to comparable single SNPs.
Conclusion: Our results suggest that ADAM17 contributes to the KD risk and is involved in secondary CAL formation via the TGF-β/SMAD3 signaling pathway. This further enriches our understanding of the importance of the signaling pathway in KD occurrence and outcomes.
What is Known: • The transforming growth factor (TGF)-β/SMAD3 signaling pathway greatly influences susceptibility to Kawasaki disease (KD) and secondary coronary artery lesions (CALs) and/or the treatment response of intravenous immunoglobulin. • A disintegrin and metalloprotease 17 (ADAM17) effectively reduces TGF-β signaling by cleaving TGF-β receptor type-1, while ADAM17 genetic variants modify human vascular pathology by differentially regulating this signaling although it is unknown whether ADAM17 contributes to KD phenotypes. |
What is New: • ADAM17 genetic variants were shown to be associated with KD risk, even when excluding the influence of TGF-β signaling pathway genes, suggesting that ADAM17 is an important KD susceptibility-related genetic locus. • The more significant compound effects of two-locus models, combining single nucleotide polymorphisms (SNPs) in ADAM17 and other TGF-β signaling pathway genes including TGFB2 and SMAD3, on KD phenotypes relative to single SNPs suggest that ADAM17 is also involved in secondary CAL formation and confers the risk of KD/CALs via the TGF-β/SMAD3 signaling pathway. |
Similar content being viewed by others
Abbreviations
- ADAM17:
-
A disintegrin and metalloprotease 17
- BLK:
-
B lymphoid kinase
- CALs:
-
Coronary artery lesions
- FCGR2A:
-
Fc fragment of IgG, low affinity IIa, receptor
- HLA:
-
Major histocompatibility complex
- IVIG:
-
Intravenous immunoglobulin
- KD:
-
Kawasaki disease
- NFAT:
-
Nuclear factor of activated T cells
- SMAD3:
-
Mothers against decapentaplegic, drosophila, homolog of, 3
- SNP:
-
Single nucleotide polymorphism
- TFBS:
-
Transcription factor binding site
- TGFB2:
-
Transforming growth factor beta-2
- TGF-β:
-
Transforming growth factor-β
- TGFBR1:
-
TGF-beta receptor type-1
- TGFBR2:
-
TGF-beta receptor type-2
References
Borzutzky A, Hoyos-Bachiloglu R, Cerda J, Talesnik E (2012) Rising hospitalization rates of Kawasaki disease in Chile between 2001 and 2007. Rheumatol Int 32:2491–2495. doi:10.1007/s00296-011-2050-4
Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, Wang XF, Frangogiannis NG (2007) Essential role of Smad3 in infarct healing and in the pathogenesis of cardiac remodeling. Circulation 116:2127–2138
Chang CJ, Kuo HC, Chang JS, Lee JK, Tsai FJ, Khor CC, Chang LC, Chen SP, Ko TM, Liu YM, Chen YJ, Hong YM, Jang GY, Hibberd ML, Kuijpers T, Burgner D, Levin M, Burns JC, Davila S, International Kawasaki Disease Genetics Consortium, Korean Kawasaki Disease Genetics Consortium, Taiwan Kawasaki Disease Genetics Consortium, Chen YT, Chen CH, Wu JY, Lee YC (2013) Replication and meta-analysis of GWAS identified susceptibility loci in Kawasaki disease confirm the importance of B lymphoid tyrosine kinase (BLK) in disease susceptibility. PLoS One 8, e72037. doi:10.1371/journal.pone.0072037
Choi YM, Shim KS, Yoon KL, Han MY, Cha SH, Kim SK, Jung JH (2012) Transforming growth factor beta receptor II polymorphisms are associated with Kawasaki disease. Korean J Pediatr 55:18–23. doi:10.3345/kjp.2012.55.1.18
Du ZD, Zhao D, Du J, Zhang YL, Lin Y, Liu C, Zhang T, Beijing Kawasaki Research Group (2007) Epidemiologic study on Kawasaki disease in Beijing from 2000 through 2004. Pediatr Infect Dis J 26:449–451
Duan J, Lou J, Zhang Q, Ke J, Qi Y, Shen N, Zhu B, Zhong R, Wang Z, Liu L, Wu J, Wang W, Gong F, Miao X (2014) A genetic variant rs1801274 in FCGR2A as a potential risk marker for Kawasaki disease: a case-control study and meta-analysis. PLoS One 9, e103329. doi:10.1371/journal.pone.0103329
Harnden A, Alves B, Sheikh A (2002) Rising incidence of Kawasaki disease in England: analysis of hospital admission data. BMJ 324:1424–1425
Holman RC, Belay ED, Christensen KY, Folkema AM, Steiner CA, Schonberger LB (2010) Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr Infect Dis J 29:483–488. doi:10.1097/INF.0b013e3181cf8705
Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, Kazue T, Eto G, Yamakawa R (1996) Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation 94:1379–1385
Kawasaki K, Freimuth J, Meyer DS, Lee MM, Tochimoto-Okamoto A, Benzinou M, Clermont FF, Wu G, Roy R, Letteboer TG, Ploos van Amstel JK, Giraud S, Dupuis-Girod S, Lesca G, Westermann CJ, Coffey RJ Jr, Akhurst RJ (2014) Genetic variants of Adam17 differentially regulate TGFβ signaling to modify vascular pathology in mice and humans. Proc Natl Acad Sci U S A 111:7723–7728. doi:10.1073/pnas.1318761111
Kawasaki T (1967) Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi 16:178–222
Khor CC, Davila S, Breunis WB, Lee YC, Shimizu C, Wright VJ, Yeung RS, Tan DE, Sim KS, Wang JJ, Wong TY, Pang J, Mitchell P, Cimaz R, Dahdah N, Cheung YF, Huang GY, Yang W, Park IS, Lee JK, Wu JY, Levin M, Burns JC, Burgner D, Kuijpers TW, Hibberd ML, Hong Kong–Shanghai Kawasaki Disease Genetics Consortium, Korean Kawasaki Disease Genetics Consortium, Taiwan Kawasaki Disease Genetics Consortium, International Kawasaki Disease Genetics Consortium, US Kawasaki Disease Genetics Consortium, Blue Mountains Eye Study (2011) Genome-wide association study identifies FCGR2A as a susceptibility locus for Kawasaki disease. Nat Genet 43:1241–1246. doi:10.1038/ng.981
Kim GB, Han JW, Park YW, Song MS, Hong YM, Cha SH, Kim DS, Park S (2014) Epidemiologic features of Kawasaki disease in South Korea: data from nationwide survey, 2009-2011. Pediatr Infect Dis J 33:24–27. doi:10.1097/INF.0000000000000010
Kuo HC, Onouchi Y, Hsu YW, Chen WC, Huang JD, Huang YH, Yang YL, Chao MC, Yu HR, Juan YS, Kuo CM, Yang KD, Huang JS, Chang WC (2011) Polymorphisms of transforming growth factor-β signaling pathway and Kawasaki disease in the Taiwanese population. J Hum Genet 56:840–845. doi:10.1038/jhg.2011.113
Lee YC, Kuo HC, Chang JS, Chang LY, Huang LM, Chen MR, Liang CD, Chi H, Huang FY, Lee ML, Huang YC, Hwang B, Chiu NC, Hwang KP, Lee PC, Chang LC, Liu YM, Chen YJ, Chen CH, Taiwan Pediatric ID Alliance, Chen YT, Tsai FJ, Wu JY (2012) Two new susceptibility loci for Kawasaki disease identified through genome-wide association analysis. Nat Genet 44:522–525. doi:10.1038/ng.2227
Li Q, Xie J, He L, Wang Y, Duan Z, Yang H, Wang Q (2015) Identification of ADAM10 and ADAM17 with potential roles in the spermatogenesis of the Chinese mitten crab, Eriocheir sinensis. Gene 562:117–127. doi:10.1016/j.gene.2015.02.060
Liu C, Xu P, Lamouille S, Xu J, Derynck R (2009) TACE-mediated ectodomain shedding of the type I TGF-beta receptor downregulates TGF-beta signaling. Mol Cell 35:26–36. doi:10.1016/j.molcel.2009.06.018
Lou J, Xu S, Zou L, Zhong R, Zhang T, Sun Y, Lu X, Liu L, Li C, Wang L, Xiong G, Wang W, Gong F, Wu J (2012) A functional polymorphism, rs28493229, in ITPKC and risk of Kawasaki disease: an integrated meta-analysis. Mol Biol Rep 39:11137–11144. doi:10.1007/s11033-012-2022-0
Lue HC, Chen LR, Lin MT, Chang LY, Wang JK, Lee CY, Wu MH (2014) Epidemiological features of Kawasaki disease in Taiwan, 1976-2007: results of five nationwide questionnaire hospital surveys. Pediatr Neonatol 55:92–96. doi:10.1016/j.pedneo.2013.07.010
Ma XJ, Yu CY, Huang M, Chen SB, Huang MR, Huang GY; Shanghai Kawasaki Research Group (2010) Epidemiologic features of Kawasaki disease in Shanghai from 2003 through 2007. Chin Med J (Engl) 123:2629–2634
Makino N, Nakamura Y, Yashiro M, Ae R, Tsuboi S, Aoyama Y, Kojo T, Uehara R, Kotani K, Yanagawa H (2015) Descriptive epidemiology of Kawasaki disease in Japan, 2011-2012: from the results of the 22nd nationwide survey. J Epidemiol 25:239–245. doi:10.2188/jea.JE20140089
Nakao A, Imamura T, Souchelnytskyi S, Kawabata M, Ishisaki A, Oeda E, Tamaki K, Hanai J, Heldin CH, Miyazono K, ten Dijke P (1997) TGF-beta receptor-mediated signalling through Smad2, Smad3 and Smad4. EMBO J 16:5353–5362
Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, Shulman ST, Bolger AF, Ferrieri P, Baltimore RS, Wilson WR, Baddour LM, Levison ME, Pallasch TJ, Falace DA, Taubert KA, Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association (2004) Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young. Pediatrics 114:1708–1733
Niu A, Wen Y, Liu H, Zhan M, Jin B, Li YP (2013) Src mediates the mechanical activation of myogenesis by activating TNFα-converting enzyme. J Cell Sci 126:4349–4357. doi:10.1242/jcs.125328
Onouchi Y (2012) Genetics of Kawasaki disease: what we know and don’t know. Circ J 76:1581–1586
Onouchi Y, Gunji T, Burns JC, Shimizu C, Newburger JW, Yashiro M, Nakamura Y, Yanagawa H, Wakui K, Fukushima Y, Kishi F, Hamamoto K, Terai M, Sato Y, Ouchi K, Saji T, Nariai A, Kaburagi Y, Yoshikawa T, Suzuki K, Tanaka T, Nagai T, Cho H, Fujino A, Sekine A, Nakamichi R, Tsunoda T, Kawasaki T, Nakamura Y, Hata A (2008) ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat Genet 40:35–42
Onouchi Y, Ozaki K, Buns JC, Shimizu C, Hamada H, Honda T, Terai M, Honda A, Takeuchi T, Shibuta S, Suenaga T, Suzuki H, Higashi K, Yasukawa K, Suzuki Y, Sasago K, Kemmotsu Y, Takatsuki S, Saji T, Yoshikawa T, Nagai T, Hamamoto K, Kishi F, Ouchi K, Sato Y, Newburger JW, Baker AL, Shulman ST, Rowley AH, Yashiro M, Nakamura Y, Wakui K, Fukushima Y, Fujino A, Tsunoda T, Kawasaki T, Hata A, Nakamura Y, Tanaka T (2010) Common variants in CASP3 confer susceptibility to Kawasaki disease. Hum Mol Genet 19:2898–2906. doi:10.1093/hmg/ddq176
Onouchi Y, Ozaki K, Burns JC, Shimizu C, Terai M, Hamada H, Honda T, Suzuki H, Suenaga T, Takeuchi T, Yoshikawa N, Suzuki Y, Yasukawa K, Ebata R, Higashi K, Saji T, Kemmotsu Y, Takatsuki S, Ouchi K, Kishi F, Yoshikawa T, Nagai T, Hamamoto K, Sato Y, Honda A, Kobayashi H, Sato J, Shibuta S, Miyawaki M, Oishi K, Yamaga H, Aoyagi N, Iwahashi S, Miyashita R, Murata Y, Sasago K, Takahashi A, Kamatani N, Kubo M, Tsunoda T, Hata A, Nakamura Y, Tanaka T, Japan Kawasaki Disease GenomeConsortium, US Kawasaki Disease Genetics Consortium (2012) A genome-wide association study identifies three new risk loci for Kawasaki disease. Nat Genet 44:517–521. doi:10.1038/ng.2220
Palazuelos J, Crawford HC, Klingener M, Sun B, Karelis J, Raines EW, Aguirre A (2014) TACE/ADAM17 is essential for oligodendrocyte development and CNS myelination. J Neurosci 34:11884–11896. doi:10.1523/JNEUROSCI.1220-14.2014
Poon CE, Madawala RJ, Day ML, Murphy CR (2015) EpCAM is decreased but is still present in uterine epithelial cells during early pregnancy in the rat: potential mechanism for maintenance of mucosal integrity during implantation. Cell Tissue Res 359:655–664. doi:10.1007/s00441-014-2017-3
Shimizu C, Jain S, Davila S, Hibberd ML, Lin KO, Molkara D, Frazer JR, Sun S, Baker AL, Newburger JW, Rowley AH, Shulman ST, Burgner D, Breunis WB, Kuijpers TW, Wright VJ, Levin M, Eleftherohorinou H, Coin L, Popper SJ, Relman DA, Fury W, Lin C, Mellis S, Tremoulet AH, Burns JC (2011) Transforming growth factor-beta signaling pathway in patients with Kawasaki disease. Circ Cardiovasc Genet 4:16–25. doi:10.1161/CIRCGENETICS.110.940858
Shimizu C, Kim J, Stepanowsky P, Trinh C, Trinh C, Lau HD, Akers JC, Chen C, Kanegaye JT, Tremoulet A, Ohno-Machado L, Burns JC (2013) Differential expression of miR-145 in children with Kawasaki disease. PLoS One 8, e58159. doi:10.1371/journal.pone.0058159
Shimizu C, Oharaseki T, Takahashi K, Kottek A, Franco A, Burns JC (2013) The role of TGF-β and myofibroblasts in the arteritis of Kawasaki disease. Hum Pathol 44:189–198. doi:10.1016/j.humpath.2012.05.004
Singh S, Vignesh P, Burgner D (2015) The epidemiology of Kawasaki disease: a global update. Arch Dis Child 100:1084–1088. doi:10.1136/archdischild-2014-307536
Villaverde-Hueso A, Alonso V, Morales-Piga A, Hens-Pérez M, Abaitua I, Posada-de-la-Paz M (2014) Childhood vasculitis hospitalizations in Spain, 1997-2011. Georgian Med News 230:65–72
Wallace CA, French JW, Kahn SJ, Sherry DD (2000) Initial intravenous gammaglobulin treatment failure in Kawasaki disease. Pediatrics 105:e78
Wu W, Misra RS, Russell JQ, Flavell RA, Rincón M, Budd RC (2006) Proteolytic regulation of nuclear factor of activated T (NFAT) c2 cells and NFAT activity by caspase-3. J Biol Chem 281:10682–10690
Xing Y, Wang H, Liu X, Yu X, Chen R, Wang C, Yu X, Sun L (2014) Meta-analysis of the relationship between single nucleotide polymorphism rs72689236 of caspase-3 and Kawasaki disease. Mol Biol Rep 41:6377–6381. doi:10.1007/s11033-014-3517-7
Yan Y, Ma Y, Liu Y, Hu H, Shen Y, Zhang S, Ma Y, Tao D, Wu Q, Peng Q, Yang Y (2013) Combined analysis of genome-wide-linked susceptibility loci to Kawasaki disease in Han Chinese. Hum Genet 132:669–680. doi:10.1007/s00439-013-1279-2
Acknowledgments
We are grateful to all the participants including the children, their parents, and the physicians who provided clinical data for the study. This study was supported by the Science & Technology Department Program of Sichuan Province (No. 2013sz0040) and the Health Department Program of Sichuan Province, China (No. 150197)
Authors’ contribution
QP cared for the patient and was responsible for gathering clinical data. HL, YY, and QP designed the study. YD performed the echocardiography of the patients with KD. QP, XY, and XL were responsible for the genetic experiment. QP performed the statistical analysis and drafted the manuscript. HL and YY made a critical revision. All of the authors discussed the content of the manuscript and approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the Ethics Committee of Clinical Trials and Biomedical Research of Sichuan University and Ethical Review Board of the Sichuan Provincial People’s Hospital, China, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Written informed consent was obtained from the parents of all the subjects who were studied prior to inclusion in the study according to the Declaration of Helsinki.
Additional information
Communicated by Jaan Toelen
Rights and permissions
About this article
Cite this article
Peng, Q., Deng, Y., Yang, X. et al. Genetic variants of ADAM17 are implicated in the pathological process of Kawasaki disease and secondary coronary artery lesions via the TGF-β/SMAD3 signaling pathway. Eur J Pediatr 175, 705–713 (2016). https://doi.org/10.1007/s00431-016-2696-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-016-2696-8