Abstract
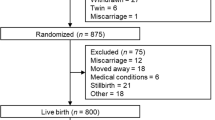
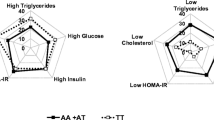
Early alterations in glucose homeostasis increase the risk of developing insulin resistance and obesity later in life. The concurrence of altered lipids and insulin sensitivity/resistance markers at birth has been scarcely investigated. The study aimed to ascertain level ranges of homocysteine (tHcyt), arylesterase (AE), lipids/lipoproteins, and insulin resistance/sensitivity markers in full-term neonates and to determine the concurrence effect of dyslipaemia and dysglycaemia on those parameters at birth. Participants were 197 full-term, 2.5 to <4.0 kg, without foetal distress Spanish newborns from the Mérida Study. Parameter percentiles for males and females were stated. The effect of the concurrence high glucose/high triglycerides (high glucose/high TG) or high glucose/low cholesterol transported by HDL (HDL-c) on tHcyt, LDL-c, HDL-c, lipoprotein (a) (Lp(a)), oxidised LDL (oxLDL), AE, glucose, insulin sensitivity (QUICKI) and insulin resistance index (HOMA-IR) was studied. Females had higher total cholesterol (TC), HDL-c, Apo A1, Lp(a) and HDL-c/Apo A1, but lower relative transport of TC (%TC) by the very low lipoprotein fraction than males. No gender differences were found for glucose, HOMA-IR and QUICKI. Neonates at the 2.5- to 2.999-kg range display more adequate HOMA-IR and QUICKI levels that their >3.0 kg counterparts. The concurrence of high glucose/high TG or high glucose/low HDL-c increased TC/HDL-c and HOMA-IR, but decreased, oxLDL, oxLDL/LDL-c and QUICKI with respect to that of low glucose/low TG or glucose/high HDL-c. The concurrence glucose/TG has predictive value for low QUICKI, whilst that of glucose/HDL-c for low QUICKI and high HOMA-IR, suggesting the importance of routine TG, HDL-c and glucose screening at birth as it would identify candidates for insulin resistance.


Similar content being viewed by others
Abbreviations
- AE:
-
Arylesterase
- Apo:
-
Apolipoproteins
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- CVD:
-
Cardiovascular disease
- DM:
-
Diabetes mellitus
- DMT2:
-
Type 2 diabetes mellitus
- HDL-c:
-
Cholesterol transported by HDL
- HOMA-IR:
-
Homeostatic model assessment—insulin resistance
- tHcyt:
-
Total homocysteine
- LDL-c:
-
Cholesterol transported by LDL
- Lp(a):
-
Lipoprotein (a)
- MS:
-
Metabolic syndrome
- oxLDL:
-
Oxidised LDL
- PON1:
-
Paraoxonase-1
- QUICKI:
-
Quantitative Insulin Sensitivity Check Index
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- VLDL:
-
Very low-density lipoproteins
- %TC:
-
Relative transport of TC
References
Aasvee K, Kurvinen E, Jordania R, Jauhiainen M, Sundvall J (2004) Lipoprotein parameters in relation to other risk factors of atherosclerosis in adults and newborns: Tallinn Young Family Study. Scand J Clin Lab Invest 64:245–253
Alexander CM, Landsman PB, Teutsch SM, Haffner SM, Third National Health and Nutrition Examination Survey (NHANES III); National Cholesterol Education Program (NCEP) (2003) NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes 52:1210–1214
Aviram M, Rosenblat M, Bisgaier CL, Newton RS, Primo-Parmo SL, La Du BN (1998) Paraoxonase inhibits high density lipoproteins oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J Clin Invest 101:1581–1590
Badiee Z, Kelishadi R (2008) Cord blood lipid profile in a population of Iranian term newborns. Pediatr Cardiol 29:574–579
Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM (1993) Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidemia (syndrome X): relation to reduced fetal growth. Diabetologia 36:62–67
Bastida S, Sánchez-Muniz FJ, Cuesta C, Perea S, Ureta A (1996) Serum apolipoproteins A-I and B in male and female full-term newborns of the Toledo-Study (Spain). Acta Paediatr 85:750–752
Bastida S, Sánchez-Muniz FJ, Cuesta C, Perea S, Aragonés A (1997) Male and female cord blood lipoprotein profile differences throughout the term-period. J Perinatal Med 27:184–191
Bastida S, Sánchez-Muniz FJ, Cuena R, Aragonés A, Bravo C (2007) Lipid and lipoprotein concentrations at age 4. Association with neonatal and parental levels. Med Clin (Barc) 128:521–528
Boyne MS, Osmond C, Fraser RA, Reid M, Taylor-Bryan C, Soares-Wynter S, Forrester TE (2010) Developmental origins of cardiovascular risk in Jamaican children: the Vulnerable Windows Cohort Study. Br J Nutr 104(7):1026–1033. doi:10.1017/S0007114510001790
Casanueva V, Cid X, Chiang MT, Molina M, Ferrada MC, Pérez R, Casanueva P (1998) Serum lipids, lipoprotein and apolipoprotein levels in normal newborns. Rev Med Chile 126:1073–1078
Couto FD, Moreira LM, Dos Santos DB, Reis MG, Gonçalves MS (2007) Folate, vitamin B12 and total homocysteine levels in neonates from Brazil. Eur J Clin Nutr 61:382–386
Gesteiro E, Bastida S, Sánchez-Muniz FJ (2009) Insulin resistance markers in term, normoweight neonates. The Mérida cohort. Eur J Pediatr 168:281–288
Gesteiro E, Bastida S, Sánchez-Muniz FJ (2011) Effects of maternal glucose tolerance, pregnancy diet quality and neonatal insulinemia upon insulin resistance/sensitivity biomarkers in normoweight neonates. Nutr Hosp 26:1447–1455
Gesteiro E, Bastida S, Vázquez-Velasco M, Corella D, Guillén M, Ordovas JM, Sánchez-Muniz FJ (2011) Effect of the S19W polymorphism in the APO A5 gene on growth, insulin resistance markers, and lipoprotein levels in normoweight neonates. Eur J Pediatr 170:1551–1558
Glueck CJ, Steiner P, Leuba U (1973) Cord blood low-density lipoprotein-cholesterol estimation versus measurement with the preparative ultracentrifuge. J Lab Clin Med 82:467–472
Hardell LI (1981) Serum lipids and lipoproteins at birth based on a study of 2815 newborn infants. Relation between materno-foetal factors and the concentration of triglycerides and cholesterol. Acta Paediatr Scand 70(Suppl 285):11–20
Hill DJ, Duvillié B (2000) Pancreatic development and adult diabetes. Pediatr Res 48:269–274
Holemans K, Aerts L, Van Assche FA (2003) Fetal growth restriction and consequences for the offspring in animal models. J Soc Gynecol Invest 10:392–399
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ (2000) Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85:2402–2410
Kirimi E, Cesur Y, Gül A (2006) Normal levels of insulin, growth hormone and cortisol levels in venous cord blood of healthy full-term infants: correlation with birthweight and placental weight. Eastern J Med 6:14–17
Leduc L, Delvin E, Ouellet A, Garofalo C, Grenier E, Morin L, Dubé J, Bouity-Voubou M, Moutquin JM, Fouron JC, Klam S, Levy E (2011) Oxidized low-density lipoproteins in cord blood from neonates with intra-uterine growth restriction. Eur J Obstet Gynecol Reprod Biol 156:46–49
Lissau I, Overpeck MD, Ruan WJ, Due P, Holstein BE, Hediger ML (2004) Body mass index and overweight in adolescents in 13 European countries, Israel, and the United States. Arch Pediatr Adolesc Med 158:27–33
Livesey G (2006) A systematic review of the glycaemic response to foods and health. ILSI Europe Workshop. Glycaemic response on health. Life Sciences Institute, Brussels, pp 82–127
Luc G, Bard J-M, Arveiler D, Ferrieres J, Evans A, Amouyel P (2002) Lipoprotein (a) as a predictor of coronary heart disease: the PRIME Study. Atherosclerosis 163:377–384
Nus M, Sánchez-Muniz FJ, Sánchez-Montero JM (2006) A new method for the determination of arylesterase activity in human serum using simulated body fluid. Atherosclerosis 188:155–159
Ong K, Kratzsch J, Kiess W, Costello M, Scott C, Dunger D (2000) Size at birth and cord blood levels of insulin, insulin-like growth factor I (IGF-I), IGF-II, IGF-binding protein-1 (IGFBP-1), IGFBP-3, and the soluble IGF-II/mannose-6-phosphate receptor in term human infants. The ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. J Clin Endocrinol Metab 85:4266–4269
Pardo MCG, Geloneze B, Tambascia MA, Bros-Filho AA (2005) Atherogenic lipid profile of Brazilian near term newborns. Brazil J Med Biol Res 38:755–760
Plagemann A (2005) Perinatal programming and functional teratogenesis: impact on body weight regulation and obesity. Physiol Behav 86:661–668
Sánchez-Muniz FJ, Cuesta Lorenzo C, Bastida Codina S, Perea Ramos S, Moya Gómez P (1994) Perfil lipoproteico en una muestra seleccionada de neonatos a término del Estudio Toledo. An Esp Pediatr 40:173–180
Srintvasan S, Berenson G (1995) Serum apolipoproteins A1 and B as markers of coronary artery disease risk early in life: the Bogalusa Heart Study. Clin Chem 41:159–164
Vázquez-Velasco M, Esperanza Díaz L, Lucas R, Gómez-Martínez S, Bastida S, Marcos A, Sánchez-Muniz FJ (2011) Effects of hydroxytyrosol-enriched sunflower oil consumption on cardiovascular disease risk factors. Br J Nutr 105:1448–1452, Corrigendum in Br J Nutr 2011;105:1712
Veeranna V, Zalawadiya SK, Niraj A, Pradhan J, Ference B, Burack RC, Jacob S, Afonso L (2011) Homocysteine and reclassification of cardiovascular disease risk. J Am Coll Cardiol 58:1025–1033
Sánchez-Muniz FJ, Gesteiro, E, Esparrago Rodilla M, Rodriguez Bernal B, Bastida S (2013) Maternal nutrition during pregnancy conditions the fetal pancreas development, hormonal status and Diabetes mellitus and metabolic syndrome biomarkers at birth. Nutr Hosp 28. doi:10.3305/nh.2013.28.2.
Wu G, Imhoff-Kunsch B, Girard AW (2012) Biological mechanisms for nutritional regulation of maternal health and fetal development. Paediatr Perinat Epidemiol 26(Suppl 1):4–26
Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, Wong G, Bennett P, Shaw J, Caprio S, IDF Consensus Group (2007) The metabolic syndrome in children and adolescents—an IDF consensus report. Ped Diabetes 8:299–306
Acknowledgments
This study was partially supported by the Spanish AGL-2008 04892-C03. Thanks are due to the Gynecology and Obstetrics Department and Laboratory Services of Mérida Hospital (Badajoz, Spain) and to participant mothers and children. We acknowledge the statistical assistance of Dras Carmen Bravo and Mar Ruperto.
Disclosure statement
The authors have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gesteiro, E., Bastida, S. & Sánchez-Muniz, F.J. Cord-blood lipoproteins, homocysteine, insulin sensitivity/resistance marker profile, and concurrence of dysglycaemia and dyslipaemia in full-term neonates of the Mérida Study. Eur J Pediatr 172, 883–894 (2013). https://doi.org/10.1007/s00431-013-1959-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-013-1959-x