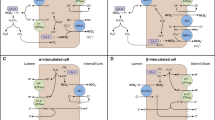
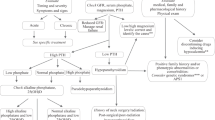
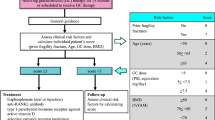
Abstract
Until a decade ago, two main hormones were recognized as directly affecting phosphate homeostasis and, with that, bone metabolism: parathyroid hormone and 1,25(OH)2 vitamin D (calcitriol). It was only a decade ago that the third major player hormone was found, linking gut, bone, and kidney. The physiologic role of fibrinogen growth factor (FGF)23 is to maintain serum phosphate concentration within a narrow range. Secreted from osteocytes, it modulates kidney handling of phosphate reabsorption and calcitriol production. Genetic and acquired abnormalities in FGF23 structure and metabolism cause conditions of either hyper-FGF23—manifested by hypophosphatemia, low serum calcitriol, and rickets/osteomalacia—or hypo-FGF23, expressed by hyperphosphatemia, high serum calcitriol, and extra-skeletal calcifications. In patients with chronic renal failure, FGF23 levels increase as kidney functions deteriorate and are under investigation to learn if the hormone actually participates in the pathophysiology of the deranged bone and mineral metabolism typical for these patients and, if so, whether it might serve as a therapeutic target. This review addresses the physiology and pathophysiology of FGF23 and its clinical applications.


Similar content being viewed by others
References
A study of KRN23 in X-linked hypophosphatemia (2010) NCT 00830675. www.clinicaltrials.gov
Alon US (2006) Hypophosphatemic vitamin D-resistant rickets. In: Primer on the metabolic bone diseases and disorders of mineral metabolism, 6th edn. American Society of Bone and Mineral Research, Washington, DC, pp 342–345
Alon US, Haney C, Moore WV (2010) Cinacalcet: a novel adjuvant treatment for XLH. Pediatr Nephrol 25:1907
Alon US, Levy-Olomucki R, Moore WV et al (2008) Calcimimetics as an adjuvant treatment for familial hypophosphatemic rickets. Clin J Am Soc Nephrol 3:658–664
Ambuhl PM, Meier D, Wolf B et al (1999) Metabolic aspects of phosphate replacement therapy for hypophosphatemia after renal transplantation: impact on muscular phosphate content, mineral metabolism, and acid/base homeostasis. Am J Kidney Dis 34:875–883
Antoniucci DM, Yamashita T, Portale AA et al (2006) Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab 91:3144–3149
Aono Y, Yamazaki Y, Yasutake J et al (2009) Therapeutic effects of anti-FGF23 antibodies in hypophosphatemic rickets/osteomalacia. J Bone Miner Res 24:1879–1888
Araya K, Fukumoto S, Backenroth R et al (2005) A novel mutation in fibroblast growth factor 23 gene as a cause of tumoral calcinosis. J Clin Endocrinol Metab 90:5523–5527
Arking DE, Becker DM, Yanek LR et al (2003) KLOTHO allele status and the risk of early-onset occult coronary artery disease. Am J Hum Genet 72:1154–1161
Arking DE, Krebsova A, Macek M et al (2002) Association of human aging with a functional variant of klotho. Proc Natl Acad Sci USA 99:856–861
Ball AM, Gillen DL, Sherrard D et al (2002) Risk of hip fracture among dialysis and renal transplant recipients. JAMA 288:3014–3018
Barsotti G, Cupisti A, Moriconi L et al (1995) Effects of reduced protein intake in rats with congenital polycystic kidney without renal failure. Contrib Nephrol 115:134–136
Basiratnia M, Fazel M, Lotfi M et al (2010) Subclinical atherosclerosis and related risk factors in renal transplant recipients. Pediatr Nephrol 25:343–348
Batsepe M, Juppner H (2008) Inherited hypophosphatemic disorders in children and the evolving mechanisms of phosphate regulation. Rev Endocr Metab Disord 9:171–180
Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V (2007) The parathyroid is a target organ for FGF23 in rats. J Clin Invest 117:4003–4008
Benet-Pages A, Orlik P, Strom TM et al (2005) An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet 14:385–390
Bergwitz C, Huppner H (2010) Regulation of phosphate homeostasis by PTH, vitamin D and FGF23. Annu Rev Med 61:91–104
Bhan I, Shah A, Holmes J et al (2006) Post-transplant hypophosphatemia: tertiary ‘hyper-phosphatoninism’? Kidney Int 70:1486–1494
Brownstein CA, Adler F, Nelson-Williams C et al (2008) A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc Natl Acad Sci USA 105:3455–3460
Cai Q, Hodgeson SF, Kao PC et al (1994) Brief report: inhibition of renal phosphate transport by a tumor product in a patient with oncogenic osteomalacia. N Engl J Med 330:1645–1649
Chalew SA, Lovchik JC, Brown et al (1996) Hypophophatemia induced in mice by transplantation of a tumor-deprived cell line from a patient with oncogenic rickets. J Pediatr Endocrinol Metab 9:593–597
Cruz EA, Lugon JR, Jorgetti V (2001) Histologic evolution of bone disease 6 months after successful kidney transplantation. Am J Kidney Dis 44:747–756
Economidou D, Dovas S, Papagianni A et al (2009) FGF-23 levels before and after renal transplantation. J Transpl 2009:379–382
Econs MJ, McEnery PT (1997) Autosomal dominant hypophosphatemic rickets/osteomalacia: clinical characterization of a novel renal phosphate-wasting disorder. J Clin Endocrinol Matab 82:674–681
Econs MJ, McEnery PT, Lennon F et al (1997) Autosomal dominant hypophosphatemic rickets is linked to chromosome 132p13. J Clin Invest 100:2653–2657
Evenepoel P, Meijers BK, de Jonge H et al (2008) Recovery of hyperphosphatoninism and renal phosphorus wasting one year after successful renal transplantation. Clin J Am Soc Nephrol 3:1829–1836
Evenepoel P, Naesens M, Claes K et al (2007) Tertiary ‘hyperphosphatoninism’ accentuates hypophosphatemia and suppresses calcitriol levels in renal transplant recipients. Am J Transplant 7:1193–1200
Eyskens B, Proesmans W, Van Damme B et al (1995) Tumour-induced rickets: a case report and review of the literature. Eur J Pediatr 154:462–464
Farrow EG, White KE (2010) Recent advances in renal phosphate handling. Nat Rev Nephrol 6:207–217
Feng JQ, Ward LM, Liu S et al (2006) Loss of DMPI causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 38:1310–1315
Ferrari SL, Bonjour JP, Rissoli R (2005) FGF-23 relationship to dietary phosphate handling in healthy young men. J Clin Endocrinol Metab 90:1519–1524
Finch JL, Tokumoto M, Nakamura H et al (2010) Effect of paricalcitol and cinacalcet on serum phosphate, FGF-23, and bone in rats with chronic kidney disease. Am J Physiol Renal Physiol 298:F1315–F1322
Fliser D, Kollerits B, Neyer U et al (2007) Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol 18:2600–2608
Garringer HJ, Fisher C, Larsson TE et al (2006) The role of mutant UDP-N-acetyl-alpha-d-galactosamine-polypeptide N-acetylgalactosaminyltransferase 3 in regulating serum intact fibroblast growth factor 23 and matrix extracellular phosphoglycoprotein in heritable tumoral calcinosis. J Clin Endocrinol Metab 91:4037–4042
Gattineni J, Bates C, Twombley K et al (2009) FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophophatemia in vivo predominately via FGF receptor 1. Am J Physiol Renal Physiol 297:F282–F291. doi:10.1152/ajprenal.90742.2008
Gattineni J, Baum M (2010) Regulation of phosphate transport by fibroblast growth factor 23 (FGF23): implications for disorders of phosphate metabolism. Pediatr Nephrol 25:591–601
Geller JL, Khosravi A, Kelly MH et al (2007) Clinacalcet in the management of tumor-induced osteomalacia. J Bone Miner Res 22:931–937
Gore MO, Welch BJ, Geng W et al (2009) Renal phosphate wasting due to tumor-induced osteomalacia: a frequently delayed diagnosis. Kidney Int 76:342–347. doi:10.1038/li.2008.355
Green J, Debby H, Lederer E et al (2001) Evidence for a PTH-independent humoral mechanism in post-transplant hypophosphatemia and phosphaturia. Kidney Int 60:1182–1196
Gupta A, Winer K, Econs MJ et al (2004) FGF-23 is elevated by chronic hyperphosphatemia. J Clin Endocrinol Metab 89:4489–4492
Gutierrez O, Isakova T, Rhee E et al (2005) Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 16:2205–2215
Gutierrez OM, Mannstadt M, Isakova T et al (2008) Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359:584–592
Habbig S, Beck BB, Feldkotter M et al (2009) Renal allograft calcification—prevalence and etiology in pediatric patients. Am J Nephrol 30:194–200
Helenius I, Remes V, Salminen S et al (2006) Incidence and predictors of fractures in children after solid organ transplantation: a 5-year prospective, population-based study. J Bone Miner Res 21:380–387
Higgins RM, Richardson AJ, Endre ZH et al (1990) Hypophosphataemia after renal transplantation: relationship to immunosuppressive drug therapy and effects on muscle detected by 31P nuclear magnetic resonance spectroscopy. Nephrol Dial Transplant 5:62–68
Hoffman WE, Hueppner HW, Deyoung BR et al (2005) Elevated fibroblast growth factor-23 in hypophosphatemic linear nevus sebaceous syndrome. Am J Med Genet 134:233–236
Ichikawa S, Imel EA, Kreiter ML et al (2007) A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest 117:2692–2701
Ito N, Fukumoto S, Takeuchi Y (2007) Effect of acute changes of serum phosphate on fibroblast (FGF)23 levels in humans. J Bone Miner Metab 25:419–422
Ix JH, Shlipak MG, Wassel CL et al (2010) Fibroblast growth factor-23 and early decrements in kidney function: the Heart and Soul Study. Nephrol Dial Transplant 25:993–997
Jonsson KB, Zahradnik R, Larsson T et al (2003) Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophophatemia. N Engl J Med 348:1656–1663
Kato K, Jeanneau C, Tarp MA (2006) Polypeptide G1NAc-transferase T3 and familial tumoral calcinosis: Secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem 281:18370–18377
Kawaguchi H, Manabe N, Miyaura C (1999) Independent impairment of osteblast and osteoclast differentiation in klotho mouse exhibiting low-turnover osteopenia. J Clin Invest 104:229–237
KDIGO CKD-MBD Work Group (2009) KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl 113:S1–S130
Kobayashi K, Imanishi Y, Koshiyama H et al (2006) Expression of FGF23 is correlated with serum phosphate level in isolated fibrous dysplasia. Life Sci 78:2295–2301
Kuro-o M (2010) Overview of the FGF23–Klotho axis. Pediatr Nephrol 25:583–590
Kuro-o M, Matsumura Y, Aizawa H (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:45–51
Kurosu H, Yamamoto M, Clark JD et al (2005) Suppression of aging in mice by the hormone Klotho. Science 309:1829–1833
Larsson T, Yu X, Davis SI et al (2008) A novel recessive mutation in fibroblast growth factor-23 causes familial tumoral calcinosis. J Clin Endocrinol Metab 90:2424–2427
Levi M (2001) Post-transplant hypophosphatemia. Kidney Int 59:2377–2387
Liu S, Tang W, Zhou J et al (2007) Distinct roles for intrinsic osteocyte abnormalities and systemic factors in regulation of FGF23 and bone mineralization in Hyp mice. Am J Physiol Endocrinol Metab 293:E1636–E1644
Lopez V, Toledo R, Sola E et al (2009) Treatment with cinacalcet in 29 kidney transplant patients with persistent hyperparathyroidism. Transplant Proc 41:2394–2395
Lorenz-Depiereux B, Bastepe M, Benet-Pages et al (2006) DMPI mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet 38:1248–1250
Lyles KW, Burkes EJ, Ellis GJ et al (1985) Genetic transmission of tumoral calcinosis: autosomal dominate with variable clinical expressivity. J Clin Endocrinol Metab 60:1093–1096
Mirza MA, Hansen T, Johansson L et al (2009) Relationship between circulating FGF23 and total body atherosclerosis in the community. Nephrol Dial Transplant 24:3125–3131
Mirza MA, Larsson A, Melhus H (2009) Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis 207:546–551
Moe SM, Chen NX, Seifert MF et al (2009) A rat model of chronic kidney disease-mineral bone disorder. Kidney Int 75:176–184
Nagano N, Miyata S, Abe M et al (2006) Effect of manipulating serum phosphorus with phosphate binder on circulating PTH and FGF23 in renal failure rats. Kidney Inter 69:425–427
Nakatani T, Sarraj B, Ohnishi M (2009) In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (FGF23)-mediated regulation of systemic phosphate homeostasis. FASEB J 23:433–441
Narayanan K, Ramachandran A, Hao J et al (2003) Dual functional roles of dentin matrix protein 1. Implications in biomineralization and gene transcription by activation of intracellular Ca2+ store. J Biol Chem 278:17500–17508
Oliveira RB, Cancela AL, Graciolli FG et al (2010) Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin J Am Soc Nephrol 5:286–291
Parfitt AM, Kleerekoper M, Cruz C (1986) Reduced phosphate reabsorption unrelated to parathyroid hormone after renal transplantation: implications for the pathogenesis of hyperparathyroidism in chronic renal failure. Miner Electrolyte Metab 12:356–362
Pattaragarn A, Alon US (2001) Antacid-induced rickets in infancy. Clin Pediatr 40:389–393
Pinheiro HS, Camara NO, Osaki KS et al (2005) Early presence of calcium oxalate deposition in kidney graft biopsies is associated with poor long-term graft survival. Am J Transplant 5:323–329
Prie D, Friedlander G (2010) Genetic disorders of renal phosphate transport. N Engl J Med 362:2399–2409
Quarles LD (2008) Endocrine functions of bone in mineral metabolism regulation. J Clin Invest 118:3820–3828
Riminucci M, Collins M, Fedark N et al (2003) FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest 112:683–692
Saito H, Kusano K, Kinosaki M (2003) Human fibroblast growth factor-23 mutants suppress Na+-dependent phosphate co-transport activity and 1alpha, 25-dihydroxyvitamin D3 production. J Biol Chem 278:2206–2211
Saito H, Maeda A, Ohtomo S (2005) Circulating FGF-23 is regulated by 1alpha,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem 280:2543–2549
Sanjad SA, Ibrahim A, Al Shorafa S et al (2001) Renal tubular dysfunction following kidney transplantation: a prospective study in 31 children. Transplant Proc 33:2830–2831
Seeherunvong W, Abitbol C, Chandar J et al (2010) Fibroblast growth factor 23 (FGF23) and left ventricular hypertrophy (LVH) in children on dialysis. Abstract submitted to the American Society of Nephrology
Serra AL, Wuhrmann C, Wuthrich RP (2008) Phosphatemic effect of cinacalcet in kidney transplant recipients with persistent hyperparathyroidism. Am J Kidney Dis 52:1151–1157
Shigematsu T, Kazama JJ, Yamashita T et al (2004) Possible involvement of circulating fibroblast growth factor 23 in the development of secondary hyperparathyroidism associated with renal insufficiency. Am J Kidney Dis 44:250–256
Shimada T, Hasegawa Y, Yamazaki Y (2004) FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostatsis. J Bone Miner Res 19:429–435
Shimada T, Kakitani M, Yamazaki Y (2004) Targeted ablation of FGF23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113:561–568
Shimada T, Mizutani S, Muto T et al (2001) Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA 98:6500–6505
Shimada T, Yamazaki Y, Takahashi M et al (2005) Vitamin D receptor-independent FGF23 actions in regulation phosphate and vitamin D metabolism. Am J Physiol Renal Physiol 289:F1088–F1095
Tenenhouse HS, Econs MH (2001) Mendelian hypophosphatemias. In: Scriver CR, Beaudet AL, Sly WS, Valley D (eds) The metabolic basis of inherited disease. McGraw-Hill, New York, pp 5039–5067
Tiosana D, Hochberg Z (2009) Hypophosphatemia: the common denominator of all rickets. J Bone Miner Metab 27:392–401
Topaz O, Shurman DL, Bergman R et al (2004) Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet 36:579–581
United States Renal Data System. Annual data report 2009, vol 2. Chapter 7: Transplantation. “http://wwwusrdsorg/2009/pdf/V2_07_09PDF” http://wwwusrdsorg/2009/pdf/V2_07_09PDF, accessed 1 June 2010
Urakawa I, Yamazaki Y, Shimada T et al (2006) Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444:770–774
Valta H, Makitie O, Ronnholm K (2009) Bone health in children and adolescents after renal transplantation. J Bone Miner Res 24:1699–1708
van Husen M, Fischer AK, Lehnhardt A et al (2010) Fibroblast growth factor 23 and bone metabolism in children with chronic kidney disease. Kidney Int 78:200–206
Weber TJ, Liu S, Indridason OS et al (2003) Serum FGF23 levels in normal and disordered phosphorus homeostasis. J Bone Miner Res 18:1227–1234
White KE, Evans WE, O’Riordan JL (2000) Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26:345–348
White KE, Evans WE, O’Riordan JLH et al (2000) Autosomal dominant hypophophatemic rickets is associated with mutations in FGF23. Nat Genet 26:345–248
Wilson AC, Greenbaum LA, Barletta GM et al (2010) High prevalence of the metabolic syndrome and associated left ventricular hypertrophy in pediatric renal transplant recipients. Pediatr Transplant 14:52–60
Wolf M (2010) Forging forward with 10 burning questions on FGF23 in kidney disease. J Am Soc Nephrol 21:427–1435
Yamamoto T, Imanishi Y, Kinoshita E et al (2005) The role of fibroblast growth factor 23 for hypophosphatemia and abnormal regulation of vitamin D metabolism in patients with McCune–Albright syndrome. J Bone Miner Metab 23:231–237
Yamashita T, Yoshioka M, Itoh N et al (2000) Identification of a novel fibroblast growth factor, FGF23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun 277:494–498
Yamazaki Y, Okazaki R, Shibata M et al (2002) Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab 87:4957–4960
Yamazaki Y, Tamada T, Kasai N et al (2008) Anti-FGF23 neutralizing antibodies show the physiological role and structural features of FGF23. J Bone Miner Res 23:1509–1518
Yu X, Sabbagh Y, Davis SI et al (2005) Genetic dissection of phosphate- and vitamin D-mediated regulation of circulation FGF23 concentrations. Bone 36:971–977
Acknowledgments
Supported by the Sam and Helen Kaplan Research Fund in Pediatric Nephrology and the Eric McClure Research Fund in Pediatric Bone and Mineral Disorders. I am thankful to Ms. Regina Johnson for her dedicated administrative assistance.
Conflict of interest
The author declares that he has no conflict of interest and no financial relationships that might have influenced the present work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alon, U.S. Clinical practice. Eur J Pediatr 170, 545–554 (2011). https://doi.org/10.1007/s00431-010-1382-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-010-1382-5