Abstract
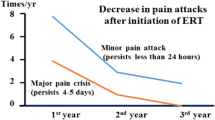
Fabry disease is an X-linked disorder of glycosphingolipid metabolism resulting from a deficiency of the lysosomal enzyme α-galactosidase A. This leads to the progressive accumulation of glycosphingolipids in lysosomes of most visceral tissues and in body fluids. Following successful clinical trials in adults, two recombinant enzyme preparations of α-galactosidase have recently been licensed in Europe for the treatment of Fabry disease and treatment in children has commenced. We now report the clinical findings and the levels of globotriaosylceramide in plasma and urine in three boys who have been treated with enzyme replacement therapy (agalsidase beta, Fabrazyme), 1 mg/kg for 2 years. In one boy there was a rapid improvement in all the clinical and biochemical parameters measured. This has been maintained. In the other two boys, who are siblings, there was no measurable clinical improvement after 1 year and the levels of globotriaosylceramide in plasma and urine, although lower than before treatment, were still considerably elevated. There was no evidence of blocking or neutralising antibodies so the dose of enzyme was increased to 2 mg/kg at 74 weeks of therapy. At 2 years their pain scores had improved but this was not accompanied by any reduction in the plasma or urine globotriaosylceramide levels. Conclusion:measurement of globotriaosylceramide in plasma and urine may not be the most appropriate marker to monitor the progression of treatment by enzyme replacement therapy in all patients. Certainly the subjective clinical improvement in the two brothers in this report outweighed the objective biochemical findings.





Similar content being viewed by others
Abbreviations
- CTH :
-
globotriasosylceramide
- ERT :
-
enzyme replacement therapy
- FA :
-
fatty acid
- LCB :
-
sphingosine
- MS/MS :
-
tandem mass spectrometry
References
Ashton-Prolla P, Tong B, Shabbeer J, Astrin KH, Eng CM, Desnick RJ (2000) Fabry disease: twenty two novel mutations in the α-galactosidase A gene and genotype/phenotype correlations in severely and mildly affected hemizygotes and heterozygotes. J Invest Med 48: 227–235
Brady RO, Gal AE, Bradley RM, Martensson E, Warshaw AL, Laster L (1967) Enzymatic defect in Fabry disease. Ceramidetrihexosidase deficiency. N Engl J Med 276: 1163–1167
Bishop DF, Calhoun DH, Bernstein HS, Hantzopoulos P, Quinn M, Desnick RJ (1986) Human alpha-galactosidase A: nucleotide sequence of a cDNA clone encoding the mature enzyme. Proc Natl Acad Sci USA 83: 4859–4863
Desnick RJ, Dawson G, Desnick SJ, Sweeley CC, Krivit W (1971) Diagnosis of glycosphingolipidoses by urinary-sediment analysis. N Engl J Med 284: 739–744
Desnick RJ, Ioannou YA, Eng CM (2001) α-Galactosidase A deficiency: Fabry disease. In: Scriver CR, Beaudet AL, Sly WS, Childs B, Kinzler KE, Vogelstein B (eds) The metabolic and molecular bases of inherited disease, 8th edn. McGraw-Hill, New York, pp 3733–3774
Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, Caplan L, Linthorst GE, Desnick RJ (2001) Safety and efficacy of recombinant human alpha-galactosidase A replacement therapy in Fabry’s disease. N Engl J Med 345: 9-16
Garman SC, Garboczi DN (2004) The molecular defect leading to Fabry disease: structure of human α-galactosidase. J Molec Biol 337: 319–335
Illsinger S, Luecke T, Langen H, Das AM (2003) Enzyme replacement therapy in an adolescent with Fabry disease. Eur J Pediatr 162: 522–523
Ishii S, Sakuraba H, Suzuki Y (1992) Point mutations in the upstream region of the alpha-galactosidase A gene exon 6 in an atypical variant of Fabry disease. Hum Genet 89: 29–32
Knol IE, Ausems MG, Lindhout D, van Diggelen OP, Verwey H, Davies J, Ploos van Amstel JK, Poll-The BT (1999) Different phenotypic expression in relatives with Fabry disease caused by a W226X mutation. Am J Med Genet 82: 436–439
Ko YH, Kim HJ, Roh YS, Park CK, Kwon CK, Park MH (1996) Atypical Fabry’s disease. An oligosymptomatic variant. Arch Pathol Lab Med 120: 86–89
Kornreich R, Bishop DF, Desnick RJ (1989) The gene encoding alpha-galactosidase A and gene rearrangements causing Fabry disease. Trans Assoc Am Phys 102: 30–43
Linhart A, Palecek T, Bultas JJ, Hrudova J, Karetova D, Zeman J, Ledvinova J, Poupetova H, Elleder M, Aschermann M (2000) New insights in cardiac structural changes in patients with Fabry’s disease. Am Heart J 139: 1101–1108
Linthorst GE, Folman CC, Aerts JM, Hollack CE (2003) Blood group does not correlate with disease severity in patients with Fabry disease (alpha-galactosidase A deficiency). Blood Cells Mol Dis 31: 324–326
MacDermot KD, Holmes A, Miners AH (2001) Anderson-Fabry disease. Clinical manifestations and impact of disease in a cohort of 60 obligate carrier females. J Med Genet 38: 769–775
Mills K, Johnson A, Winchester B (2002) Synthesis of novel internal standards for the quantitative determination of plasma ceramide trihexoside in Fabry disease by tandem mass spectrometry. FEBS Lett 515: 171–176
Nagao Y, Nakashima H, Fukuhara Y, Shimmoto M, Oshima A, Ikari Y, Mori Y, Sakuraba H, Suzuki Y (1991) Hypertrophic cardiomyopathy in late-onset variant of Fabry disease with high residual activity of alpha-galactosidase A. Clin Genet 39: 233–237
Okumiya T, Kawamura O, Itoh K, Kase R, Ishii S, Kamei S, Sakuraba H (1998) Novel missense mutation (M72V) of alpha-galactosidase gene and its expression product in an atypical Fabry hemizygote. Hum Mutat [Suppl 1]: S213–S216
Ries M, Ramaswani U, Parini R, Lindblad B, Whybra C, Willers I, Gal A, Beck M (2003) The early clinical phenotype of Fabry disease: a study of 35 European children and adolescents. Eur J Pediatr 162: 767–772
Sakuraba H, Oshima A, Fukuhara Y, Shimmoto M, Nagao Y, Bishop DF, Desnick RJ, Suzuki Y (1990) Identification of point mutations in the alpha-galactosidase A gene in classical and atypical hemizygotes with Fabry disease. Am J Hum Genet 47: 784–789
Schiffmann R, Scott LJC (2002) Pathophysiology and assessment of neuropathic pain in Fabry disease. Acta Paediatr Suppl 439: 48–52
Schiffmann R, Kopp JB, Austin III HA, Sabnis S, Moore DF, Weibel T, Balow JE, Brady RO (2001) Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA 285: 2743–2749
Wherrett JR, Hakomori SI (1973) Characterization of a blood group B glycolipid accumulating in the pancreas of a patient with Fabry’s disease. J Biol Chem 248: 3046–3051
Wilcox WR; Banikazemi M, Guffon N, Lee P, Linthorst GE, Waldek S, Germain D, Desnick RJ for the International Collaborative Group on Fabry Disease (2003) Long term enzyme replacement therapy for Fabry disease (abstract). Am J Hum Genet 73: S5 624
Acknowledgements
We thank the Genzyme Corporation and the Fabry Support Group UK for financial support and the staff of the Enzyme Laboratory at Great Ormond Street Hospital for their help with the samples. Mutational analysis was done in the Clinical Molecular Genetics Laboratory at Great Ormond Street Hospital. Research at the Institute of Child Health and Great Ormond Street Hospital NHS Trust benefits from research and development funding received from the National Health Service Executive.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mills, K., Vellodi, A., Morris, P. et al. Monitoring the clinical and biochemical response to enzyme replacement therapy in three children with Fabry disease. Eur J Pediatr 163, 595–603 (2004). https://doi.org/10.1007/s00431-004-1484-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-004-1484-z