Abstract
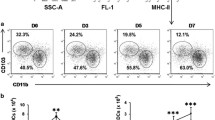
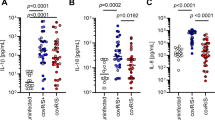
Infection with Burkholderia cepacia complex (Bcc) bacteria is a threat to cystic fibrosis (CF) patients, commonly leading to a fatal pneumonia, the cepacia syndrome. It causes a massive production of pro-inflammatory cytokines and leucocyte recruitment to airway epithelium without resolving infection and contributing to tissue lesion. To dissect how Bcc bacteria subvert the immune response, we developed a co-culture model with human dendritic cells (DCs) and B. cenocepacia clonal variants isolated from a chronically infected CF patient, who died with cepacia syndrome. We demonstrated that the two late variants were sevenfold and 17-fold (respectively) more internalized by DCs than the variant that initiated infection. The late variants showed improved survival within DCs (60.29 and 52.82 CFU/DC) compared to the initial variant (0.38 CFU/DC). All clonal isolates induced high expression of inflammatory cytokines IL-8, IL-6, IL-1β, IL-12, IL-23, TNF-α and IL-1β. This pro-inflammatory trait was significantly more pronounced in DCs infected with the late variants than in DCs infected with the variant that initiated patient’s infection. All infected DCs failed to upregulate maturation markers, HLA-DR, CD80, CD86 and CD83. Nevertheless, these infected DCs activated approximately twice more T cells than non-infected DCs. Similar T cell activation was observable with respective conditioned media, suggesting a non-antigen-specific activation. Our data indicate that during prolonged infection, B. cenocepacia acquires ability to survive intracellularly, inducing inflammation, while refraining DC’s maturation and stimulating non-antigen-specific T cell responses. The co-culture model here developed may be broadly applied to study B. cenocepacia-induced immunomodulation.






Similar content being viewed by others
References
Winkelstein JA, Marino MC, Johnston RB Jr, Boyle J, Curnutte J, Gallin JI et al (2000) Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine 79(3):155–169
Mahenthiralingam E, Baldwin A, Dowson CG (2008) Burkholderia cepacia complex bacteria: opportunistic pathogens with important natural biology. J Appl Microbiol 104(6):1539–1551
Drevinek P, Mahenthiralingam E (2010) Burkholderia cenocepacia in cystic fibrosis: epidemiology and molecular mechanisms of virulence. Clin Microbiol Infect 16(7):821–830. doi:10.1111/j.14690691.2010.03237.x
Döring G, Parameswaran IG, Murphy TF (2011) Differential adaptation of microbial pathogens to airways of patients with cystic fibrosis and chronic obstructive pulmonary disease. FEMS Microbiol Rev 35(1):124–146. doi:10.1111/j.1574-6976.2010.00237.x
Moreira AS, Mil-Homens D, Sousa SA, Coutinho CP, Pinto-de-Oliveira A, Ramos CG, dos Santos SC, Fialho AM, Leitão JH, Sá-Correia I (2011) Variation of Burkholderia cenocepacia virulence potential during cystic fibrosis chronic lung infection. Virulence. doi:10.1080/21505594.2016.1237334
Lieberman TD, Michel JB, Aingaran M, Potter-Bynoe G, Roux D, Davis MR et al (2011) Parallel bacterial evolution within multiple patients identifies candidate pathogenicity genes. Nat Genet 43(12):1275–1280. doi:10.1038/ng.997
Saldias MS, Valvano MA (2009) Interactions of Burkholderia cenocepacia and other Burkholderia cepacia complex bacteria with epithelial and phagocytic cells. Microbiology (Reading, England) 155(Pt 9):2809–2817
Lamothe J, Huynh KK, Grinstein S, Valvano MA (2007) Intracellular survival of Burkholderia cenocepacia in macrophages is associated with a delay in the maturation of bacteria-containing vacuoles. Cell Microbiol 9(1):40–53
Keith KE, Hynes DW, Sholdice JE, Valvano MA (2009) Delayed association of the NADPH oxidase complex with macrophage vacuoles containing the opportunistic pathogen Burkholderia cenocepacia. Microbiology (Reading, England) 155(Pt 4):1004–1015
Hespel C, Moser M (2012) Role of inflammatory dendritic cells in innate and adaptive immunity. Eur J Immunol 42(10):2535–2543. doi:10.1002/eji.201242480
Palucka K, Banchereau J (2002) How dendritic cells and microbes interact to elicit or subvert protective immune responses. Curr Opin Immunol 14(4):420–431
Schmidt SV, Nino-Castro AC, Schultze JL (2012) Regulatory dendritic cells: there is more than just immune activation. Front Immunol 3:274. doi:10.3389/fimmu.2012.00274
Xu Y, Tertilt C, Krause A, Quadri LE, Crystal RG, Worgall S (2009) Influence of the cystic fibrosis transmembrane conductance regulator on expression of lipid metabolism-related genes in dendritic cells. Respir Res 10:26. doi:10.1186/1465-9921-10-26
Roghanian A, Drost EM, MacNee W, Howie SE, Sallenave JM (2006) Inflammatory lung secretions inhibit dendritic cell maturation and function via neutrophil elastase. Am J Respir Crit Care Med 174(11):1189–1198. doi:10.1164/rccm.200605-632OC
MacDonald KL, Speert DP (2008) Differential modulation of innate immune cell functions by the Burkholderia cepacia complex: Burkholderia cenocepacia but not Burkholderia multivorans disrupts maturation and induces necrosis in human dendritic cells. Cell Microbiol 10(10):2138–2149
Richau JA, Leitão JH, Correia M, Lito L, Salgado MJ, Barreto C et al (2000) Molecular typing and exopolysaccharide biosynthesis of Burkholderia cepacia isolates from a Portuguese cystic fibrosis center. J Clin Microbiol 38(4):1651–1655
Cunha MV, Leitão JH, Mahenthiralingam E, Vandamme P, Lito L, Barreto C et al (2003) Molecular analysis of Burkholderia cepacia complex isolates from a Portuguese cystic fibrosis center: a 7-year study. J Clin Microbiol 41(9):4113–4120
Coutinho CP, Dos Santos SC, Madeira A, Mira NP, Moreira AS, Sá-Correia I (2011) Long-term colonization of the cystic fibrosis lung by Burkholderia cepacia complex bacteria: epidemiology, clonal variation, and genome-wide expression alterations. Front Cell Infect Microbiol 1:12. doi:10.3389/fcimb.2011.00012
Mira NP, Madeira A, Moreira AS, Coutinho CP, Sá-Correia I (2011) Genomic expression analysis reveals strategies of Burkholderia cenocepacia to adapt to cystic fibrosis patients’ airways and antimicrobial therapy. PLoS ONE 6(12):e28831. doi:10.1371/journal.pone.0028831
Madeira A, Santos PM, Coutinho CP, Pinto-de-Oliveira A, Sá-Correia I (2011) Quantitative proteomics (2-D DIGE) reveals molecular strategies employed by Burkholderia cenocepacia to adapt to the airways of cystic fibrosis patients under antimicrobial therapy. Proteomics 11(7):1313–1328. doi:10.1002/pmic.201000457
Madeira A, dos Santos SC, Santos PM, Coutinho CP, Tyrrell J, McClean S et al (2013) Proteomic profiling of Burkholderia cenocepacia clonal isolates with different virulence potential retrieved from a cystic fibrosis patient during chronic lung infection. PLoS ONE 8(12):e83065. doi:10.1371/journal.pone.0083065
Videira PA, Amado IF, Crespo HJ, Alguero MC, Dall’Olio F, Cabral MG et al (2008) Surface alpha 2-3- and alpha 2-6-sialylation of human monocytes and derived dendritic cells and its influence on endocytosis. Glycoconj J 25(3):259–268
Cabral MG, Silva Z, Ligeiro D, Seixas E, Crespo H, Carrascal MA et al (2013) The phagocytic capacity and immunological potency of human dendritic cells is improved by α2,6-sialic acid deficiency. Immunology 138(3):235–245. doi:10.1111/imm.12025
Videira PA, Piteira AR, Cabral MG, Martins C, Correia M, Severino P et al (2011) Effects of bevacizumab on autocrine VEGF stimulation in bladder cancer cell lines. Urol Int 86(1):95–101. doi:10.1159/000321905
Videira PA, Correia M, Malagolini N, Crespo HJ, Ligeiro D, Calais FM et al (2009) ST3Gal. I sialyltransferase relevance in bladder cancer tissues and cell lines. BMC Cancer 9:357. doi:10.1186/1471-2407-9-357
Crespo HJ, Cabral MG, Teixeira AV, Lau JTY, Trindade H, Videira PA (2009) Effect of sialic acid loss on dendritic cell maturation. Immunology 128(1):e621–e631. doi:10.1111/j.1365-2567.2009.03047.x
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392(6673):245–252. doi:10.1038/32588
Simms PE, Ellis TM (1996) Utility of flow cytometric detection of CD69 expression as a rapid method for determining poly- and oligoclonal lymphocyte activation. Clin Diagn Lab Immunol 3(3):301–304
Zhang J, Jiang R, Wang W, Takayama H, Tanaka Y (2013) Apoptosis are induced in J774 macrophages upon phagocytosis and killing of Pseudomonas aeruginosa. Cell Immunol 286(1–2):11–15. doi:10.1016/j.cellimm.2013.10.006
Keith KE, Valvano MA (2007) Characterization of SodC, a periplasmic superoxide dismutase from Burkholderia cenocepacia. Infect Immun 75(5):2451–2460
Huynh KK, Plumb JD, Downey GP, Valvano MA, Grinstein S (2010) Inactivation of macrophage Rab7 by Burkholderia cenocepacia. J Innate Immun 2(6):522–533. doi:10.1159/000319864
Sajjan US, Yang JH, Hershenson MB, LiPuma JJ (2006) Intracellular trafficking and replication of Burkholderia cenocepacia in human cystic fibrosis airway epithelial cells. Cell Microbiol 8(9):1456–1466. doi:10.1111/j.1462-5822.2006.00724.x
Reddi K, Phagoo SB, Anderson KD, Warburton D (2003) Burkholderia cepacia-induced IL-8 gene expression in an alveolar epithelial cell line: signaling through CD14 and mitogen-activated protein kinase. Pediatr Res 54(3):297–305. doi:10.1203/01.PDR.0000076661.85928.1D
Kaza SK, McClean S, Callaghan M (2011) IL-8 released from human lung epithelial cells induced by cystic fibrosis pathogens Burkholderia cepacia complex affects the growth and intracellular survival of bacteria. Int J Med Microbiol 301(1):26–33. doi:10.1016/j.ijmm.2010.06.005
Harris WT, Muhlebach MS, Oster RA, Knowles MR, Clancy JP, Noah TL (2011) Plasma TGF-β1 in pediatric cystic fibrosis: potential biomarker of lung disease and response to therapy. Pediatr Pulmonol 46(7):688–695. doi:10.1002/ppul.21430
Isomäki P, Clark JM, Panesar M, Cope AP (2005) Pathways of T cell activation and terminal differentiation in chronic inflammation. Curr Drug Targets Inflamm Allergy 4(3):287–293
Acknowledgements
This work was supported by the Portuguese Foundation for Science and Technology (FCT)—PTDC/SAU-MII/67561/2006 (Paula A. Videira). Funding received by iBB - Institute for Bioengineering and Biosciences from Programa Operacional Regional de Lisboa 2020 (Project N. 007317) and from the FCT (UID/BIO/04565/2013) is acknowledged. FCT also supported postdoctoral fellowship to Carla P. Coutinho (SFRH/BPD/81220/2011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Peripheral blood of healthy volunteers was anonymously provided by IPST according to its ethical guidelines—No5/GDG (21/06/2011). The study was approved by Faculdade de Ciências Médicas Ethics Committee.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Human and animal rights
This article does not contain any studies with animals performed by any of the authors.
Additional information
M. Guadalupe Cabral and Marília Pereira have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guadalupe Cabral, M., Pereira, M., Silva, Z. et al. Using dendritic cells to evaluate how Burkholderia cenocepacia clonal isolates from a chronically infected cystic fibrosis patient subvert immune functions. Med Microbiol Immunol 206, 111–123 (2017). https://doi.org/10.1007/s00430-016-0488-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-016-0488-4