Abstract
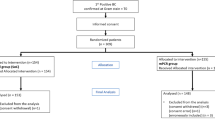
Multiplex PCR (mPCR) directly from blood has been suggested as a promising method for rapid identification of pathogens causing sepsis. This study aimed to investigate whether mPCR has any impact on antimicrobial treatment. Hematological patients with febrile neutropenia were randomized into two groups. In the study group, mPCR was performed as an addition to standard diagnostics, and PCR finding was immediately communicated to the clinicians, thus being available for decision making. In the control group, clinicians were not aware of PCR result. PCR samples were collected simultaneously with clinically indicated blood culture specimens from peripheral vein and/or central venous catheter at fever onset and once again if fever persisted up to 72 h. Overall, 74 patients of the study group and 76 patients of the control group were enrolled and 253 samples collected. Therapy was changed to targeted antimicrobial therapy (AMT) in 12 patients (16.2 %) in the study group and in 12 patients (15.8 %) in the control group. For patients with changes, the median time to change to the targeted AMT was 21.4 h in the study group and 47.5 h in the control group (p = 0.018). In the study group, 57.1 % (8/14) of changes to targeted AMT was due to PCR finding. PCR led to AMT change in 9.5 % (7/74) of study group patients, i.e., in 33.3 % (7/21) of patients who had positive PCR finding. There were no significant differences in patient outcomes (secondary endpoints). In conclusion, PCR method accelerates change to the targeted AMT in febrile neutropenic patients.


Similar content being viewed by others
References
Penack O, Buchheidt D, Christopeit M, von Lilienfeld-Toal M, Massenkeil G, Hentrich M, Salwender H, Wolf HH, Ostermann H (2011) Management of sepsis in neutropenic patients: guidelines from the infectious diseases working party of the German Society of Hematology and Oncology. Ann Oncol 22:1019–1029
Donowitz GR, Maki DG, Crnich CJ, Pappas PG, Rolston KV (2001) Infections in the neutropenic patient—new views of an old problem. Hematology Am Soc Hematol Educ Program 2001:113–139
Sharma A, Lokeshwar N (2005) Febrile neutropenia in haematological malignancies. J Postgrad Med 51(Suppl 1):S42–S48
Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M (2006) Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34:1589–1596
Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE, Dodek P, Wood G, Kumar A, Simon D, Peters C, Ahsan M, Chateau D (2009) Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 136:1237–1248
von Lilienfeld-Toal M, Lehmann LE, Raadts AD, Hahn-Ast C, Orlopp KS, Marklein G, Purr I, Cook G, Hoeft A, Glasmacher A, Stuber F (2009) Utility of a commercially available multiplex real-time PCR assay to detect bacterial and fungal pathogens in febrile neutropenia. J Clin Microbiol 47:2405–2410
Lamoth F, Jaton K, Prod’hom G, Senn L, Bille J, Calandra T, Marchetti O (2010) Multiplex blood PCR in combination with blood cultures for improvement of microbiological documentation of infection in febrile neutropenia. J Clin Microbiol 48:3510–3516
Lehmann LE, Hunfeld KP, Emrich T, Haberhausen G, Wissing H, Hoeft A, Stuber F (2008) A multiplex real-time PCR assay for rapid detection and differentiation of 25 bacterial and fungal pathogens from whole blood samples. Med Microbiol Immunol 197:313–324
Bloos F, Hinder F, Becker K, Sachse S, Mekontso DA, Straube E, Cattoir V, Brun-Buisson C, Reinhart K, Peters G, Bauer M (2010) A multicenter trial to compare blood culture with polymerase chain reaction in severe human sepsis. Intensive Care Med 36:241–247
Dark PM, Dean P, Warhurst G (2009) Bench-to-bedside review: the promise of rapid infection diagnosis during sepsis using polymerase chain reaction-based pathogen detection. Crit Care 13:217
Bloos F, Sachse S, Kortgen A, Pletz MW, Lehmann M, Straube E, Riedemann NC, Reinhart K, Bauer M (2012) Evaluation of a polymerase chain reaction assay for pathogen detection in septic patients under routine condition: an observational study. PLoS ONE 7:e46003
Pletz MW, Wellinghausen N, Welte T (2011) Will polymerase chain reaction (PCR)-based diagnostics improve outcome in septic patients? A clinical view. Intensive Care Med 37:1069–1076
Avolio M, Diamante P, Zamparo S, Modolo ML, Grosso S, Zigante P, Tosoni N, De Rosa R, Stano P, Camporese A (2010) Molecular identification of bloodstream pathogens in patients presenting to the emergency department with suspected sepsis. Shock 34:27–30
Dierkes C, Ehrenstein B, Siebig S, Linde HJ, Reischl U, Salzberger B (2009) Clinical impact of a commercially available multiplex PCR system for rapid detection of pathogens in patients with presumed sepsis. BMC Infect Dis 9:126
Lehmann LE, Alvarez J, Hunfeld KP, Goglio A, Kost GJ, Louie RF, Raglio A, Regueiro BJ, Wissing H, Stuber F (2009) Potential clinical utility of polymerase chain reaction in microbiological testing for sepsis. Crit Care Med 37:3085–3090
Maubon D, Hamidfar-Roy R, Courby S, Vesin A, Maurin M, Pavese P, Ravanel N, Bulabois CE, Brion JP, Pelloux H, Timsit JF (2010) Therapeutic impact and diagnostic performance of multiplex PCR in patients with malignancies and suspected sepsis. J Infect 61:335–342
Bravo D, Blanquer J, Tormo M, Aguilar G, Borras R, Solano C, Clari MA, Costa E, Munoz-Cobo B, Argueso M, Pineda JR, Navarro D (2011) Diagnostic accuracy and potential clinical value of the LightCycler SeptiFast assay in the management of bloodstream infections occurring in neutropenic and critically ill patients. Int J Infect Dis 15:e326–e331
Grif K, Fille M, Wurzner R, Weiss G, Lorenz I, Gruber G, Eschertzhuber S, Nachbaur D, Lass-Florl C, Orth D (2012) Rapid detection of bloodstream pathogens by real-time PCR in patients with sepsis. Wien Klin Wochenschr 124:266–270
Lodes U, Bohmeier B, Lippert H, Konig B, Meyer F (2012) PCR-based rapid sepsis diagnosis effectively guides clinical treatment in patients with new onset of SIRS. Langenbecks Arch Surg 397:447–455
Wallet F, Nseir S, Baumann L, Herwegh S, Sendid B, Boulo M, Roussel-Delvallez M, Durocher AV, Courcol RJ (2010) Preliminary clinical study using a multiplex real-time PCR test for the detection of bacterial and fungal DNA directly in blood. Clin Microbiol Infect 16:774–779
Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA, Wingard JR, Infectious Diseases Society of America (2011) Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 52:e56–e93
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101:1644–1655
Seifert H, Abele-Horn M, Fätkenheuer G, Glück T, Jansen B, Kern W, Mack D, Plum G, Reinert RR, Roos R, Salzberger B, Shah PM, Ullmann U, Weiss M, Welte T, Wisplinghoff H (2007) Part 3: Blutkulturdiagnostik–Sepsis, Endokarditis, Katheterinfektionen. In: Mauch H, Podbielski A, Herrmann M (eds) MIQ: Qualitätsstandards in der mikrobiologisch-infektiologischen Diagnostik, 2nd edn. Elsevier, Urban and Fischer, Munich
Becker K, von Eiff C (2011) Staphylococcus, Micrococcus, and other catalase-positive cocci. In: Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW (eds) Manual of clinical microbiology, 10th edn. ASM press, Washington, pp 308–330
Spellerberg B, Brandt C (2011) Streptococcus. In: Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW (eds) Manual of clinical microbiology, 10th edn. ASM press, Washington, pp 331–349
Wauters G, Vaneechoutte M (2011) Approaches to the identification of aerobic Gram-negative bacteria. In: Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW (eds) Manual of clinical microbiology, 10th edn. ASM press, Washington, pp 539–558
Roche Diagnostics GmbH (2011) LightCycler® SeptiFast Test MGrade Package Insert 04623886001-06. Mannheim, Germany
Kerremans JJ, Verboom P, Stijnen T, Hakkaart-van RL, Goessens W, Verbrugh HA, Vos MC (2008) Rapid identification and antimicrobial susceptibility testing reduce antibiotic use and accelerate pathogen-directed antibiotic use. J Antimicrob Chemother 61:428–435
Patel R (2013) Matrix-assisted laser desorption ionization-time of flight mass spectrometry in clinical microbiology. Clin Infect Dis 57:564–572
Clerc O, Prod’hom G, Vogne C, Bizzini A, Calandra T, Greub G (2013) Impact of matrix-assisted laser desorption ionization time-of-flight mass spectrometry on the clinical management of patients with Gram-negative bacteremia: a prospective observational study. Clin Infect Dis 56:1101–1107
Struelens MJ (2010) Detection of microbial DNAemia: does it matter for sepsis management? Intensive Care Med 36:193–195
Acknowledgments
This study was part of a joint project of a Münster-Berlin–Munich Molecular Diagnostics of Sepsis Study Group aiming at improvement of diagnostics, AMT and pharmaco-economics of sepsis. The authors are indebted to all members of the study group not listed as authors, including M. Deja, Berlin, C. Spies, Berlin, H. Bunzemeier, Münster, R. Grube, Munich, J. Schulze, Berlin, D. Voss, Münster and M. Wilke, Munich. The authors are thankful to M. Schulte, M. Reher and J. Radtke for excellent technical assistance and to the staff of the Department of Medicine A, University Hospital Münster, for support. This work was partly supported by Roche Diagnostics and Pfizer, Germany. Roche Diagnostics provided SeptiFast kits and made LightCycler 2.0 available at the study site for the study period. Pfizer provided a restricted grant for study activities.
Conflict of interest
Lecture fees were received from Pfizer (EAI, GS, YN, ST, IN, WEB) and Roche Diagnostics (ST, IN, WEB). KB received lecture fees, grants and travel support from Pfizer and Roche Diagnostics. All other authors report no conflict of interest.
Ethical standard
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Evgeny A. Idelevich and Gerda Silling have contributed equally to this work.
ClinicalTrials.gov registration NCT01114165.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Idelevich, E.A., Silling, G., Niederbracht, Y. et al. Impact of multiplex PCR on antimicrobial treatment in febrile neutropenia: a randomized controlled study. Med Microbiol Immunol 204, 585–592 (2015). https://doi.org/10.1007/s00430-014-0385-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-014-0385-7