Abstract
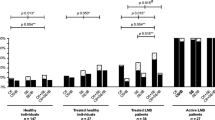
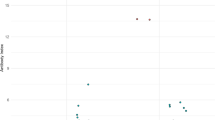
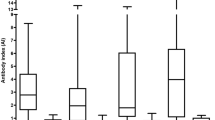
Detection of intrathecally produced specific antibodies (AI) is essential in the diagnosis of Lyme neuroborreliosis (LNB); however, the performance of various newer AI detection methods has not been systematically assessed. Here we assessed and compared advanced test systems for detecting borrelia IgG-AI and IgM-AI. Serum and cerebrospinal fluid (CSF) samples from well-defined LNB and tick-borne encephalitis (TBE) patients, 25 each, were tested with three antibody detection systems, one based on chemiluminescence (CLA) and two based on enzyme-linked immunosorbent assays (ELISA), employing different antigens for detection of IgG and IgM antibodies. In samples from patients with LNB, IgG-AI was detected in 20 samples by CLA, 19 by ELISA1, and 22 by ELISA2, and IgM-AI was detected in 16 samples by CLA, six by ELISA1, and 11 by ELISA2. In samples from TBE patients, IgG-AI was positive in one case by CLA and ELISA2, and in 7 cases by ELISA1, whereas IgM-AI was positive in one case by CLA and in none by ELISA. IgG-AI and IgM-AI were not detected within the first week of disease. Duration of disease correlated with IgG-AI while IgM-AI results were heterogeneous for each test assay. Moreover, the levels of IgG-AI, but not IgM-AI, correlated with protein concentration in CSF. IgG is the relevant immunoglobulin isotype for detecting intrathecal synthesis of borrelia antibodies. The highest sensitivity and specificity were achieved by the antibody detection assay using VlsE IR6 peptide. Detection of IgM-AI yielded heterogenous results and did not support the laboratory diagnosis of LNB.




Similar content being viewed by others
References
Berglund J, Eitrem R, Ornstein K et al (1995) An epidemiologic study of Lyme disease in southern Sweden. N Engl J Med 333:1319–1327
Stanek G, Wormser GP, Gray J, Strle F (2012) Lyme borreliosis. Lancet 2012(379):461–473. doi:10.1016/S0140-6736(11)60103-7
Stanek G, Fingerle V, Hunfeld KP et al (2011) Lyme borreliosis: clinical case definitions for diagnosis and management in Europe. Clin Microbiol Infect 17:69–79. doi:10.1111/j.1469-0691.2010.03175.x
Strle F, Stanek G (2009) Clinical manifestations and diagnosis of Lyme borreliosis. Curr Probl Dermatol 37:51–110. doi:10.1097/QCO.0b013e32832ee880
Mygland A, Ljostadt U, Fingerle V et al (2010) EFNS guidelines on the diagnosis and management of European Lyme neuroborreliosis. Eur J Neurol 17:8–16. doi:10.1111/j.1468-1331.2009.02862.x
Hubálek Z (2009) Epidemiology of Lyme borreliosis. Curr Probl Dermatol 37:31–50. doi:10.1159/000213069
Wilske B, Schierz G, Preac-Mursic V et al (1986) 1986) Intrathecal production of specific antibodies against Borrelia burgdorferi in patients with lymphocytic meningoradiculitis (Bannwarth’s syndrome. J Infect Dis 153:304–314
Hansen K (1994) Lyme neuroborreliosis: improvements of the laboratory diagnosis and a survey of epidemiological and clinical features in Denmark 1985–1990. Acta Neurol Scand 89(Suppl 151):1–44
Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP (2005) Diagnosis of Lyme borreliosis. Clin Microbiol Rev 18:484–509
Cerar T, Ogrinc K, Strle F, Ružić-Sabljić E (2010) Humoral immune responses in patients with Lyme neuroborreliosis. Clin Vaccine Immunol 17:645–650. doi:10.1128/CVI.00341-09
Kaiser R, Lücking CH (1993) Intrathecal synthesis of specific antibodies in neuroborreliosis. Comparison of different ELISA techniques and calculation methods. J Neurol Sci 118:64–72
Ružić-Sabljić E, Maraspin V, Cimperman J, Lotrič-Furlan S, Strle F (2002) Evaluation of immunofluorescence test (IFT) and immune Western blot (WB) test in patients with erythema migrans. Wien Klin Wochenschr 114:586–590
Reiber H, Peter JB (2001) Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J Neurol Sci 184:101–122
R Development Core Team (2009) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL. http://www.R-project.org. Accessed May 31st, 2013
Kaiser R, Rauer S (1998) Analysis of the intrathecal immune response in neuroborreliosis to a sonicate antigen and three recombinant antigens of Borrelia burgdorferi sensu stricto. Eur J Clin Microbiol Infect Dis 17:159–166
Ljostad U, Skarpaas T, Mygland A (2007) Clinical usefulness of intrathecal antibody testing in acute Lyme neuroborreliosis. Eur J Neurol 14:873–876
Tumani H, Nölker G, Reiber H (1995) Relevance of cerebrospinal fluid variables for early diagnosis of neuroborreliosis. Neurology 45:1663–1670
Tjernberg I, Henningsson AJ, Eliasson I, Forsberg P, Ernerudh J (2011) Diagnostic performance of cerebrospinal fluid chemokine CXCL13 and antibodies to the C6-peptide in Lyme neuroborreliosis. J Infect 62:149–158
Acknowledgments
We are grateful to Brigitte Zinke for her excellent technical assistance. We thank the companies Diasorin Austria, Euroimmun Germany, and Medac Germany for donating the test kits for this study (LIAISON Borrelia IgG/IgMquant, Diasorin, Salugga, Italy, Lot Numbers: 089039/3 [IgG], 124023/1 [IgM]; Anti-Borrelia-plus-VlsE-ELISA [IgG], andAnti-Borrelia-ELISA [IgM], Euroimmun Medizinische Labordiagnostika AG, Lübeck, Germany, Lot Numbers: E110215AH [IgG], E110215AI [IgM]; and, Borrelia-IgG-/-IgM-ELISAmedac, Medac GmbH, Hamburg, Germany, Lot numbers: BORG05 [IgG], BORM04 [IgM]). FS received funding from Slovenian Research Agency (Grantp3-0296).
Conflict of interest
The authors FS, GS, and MM are members of the ESCMID study group on Lyme borreliosis, ESGBOR. The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Stanek, G., Lusa, L., Ogrinc, K. et al. Intrathecally produced IgG and IgM antibodies to recombinant VlsE, VlsE peptide, recombinant OspC and whole cell extracts in the diagnosis of Lyme neuroborreliosis. Med Microbiol Immunol 203, 125–132 (2014). https://doi.org/10.1007/s00430-013-0322-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-013-0322-1